
The reaction can be classified as:
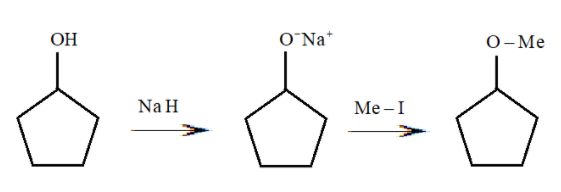
A) Williamson ether synthesis reaction
B) Alcohol formation reaction
C) Dehydration reaction
D) Williamson alcohol synthesis reaction

Answer
562.8k+ views
Hint: The ‘Me’ group used is the reaction is the methyl group. As we can see that the product formed in the reaction is not an alcohol so straightaway, we can say that this is not an alcohol formation reaction and nor is it a dehydration reaction as no water molecule is being released.
Complete answer:
According to our question, the correct answer is Williamson ether synthesis reaction as it involves the treatment of sodium alkoxide with a suitable alkyl halide to form ether. Here we see that cyclopentanol reacts with sodium hydride to form sodium salt of cyclopentolate. After that it reacts with methyl iodide to form an ether named methylcyclopentane.
Williamson Ether Synthesis usually takes place by a \[{S_N}2\] reaction mechanism where we see that a primary alkyl halide reacts with an alkoxide ion. \[{S_N}2\] Pathway is required for the synthesis of this reaction. The alkyl halide is usually primary or secondary. The Ethers delivered in this manner have more carbon ions than both of the beginning materials and accordingly are more perplexing structures.
Therefore, the correct answer is option A.
Note: Alkoxide ions are good nucleophiles and displace halide ions from alkyl halides resulting in the formation of a new carbon oxygen bond. Alkoxide is produced by treatment of alcohols with either a base or an alkali metal. This method is considered the best for the preparation of ethers.
Complete answer:
According to our question, the correct answer is Williamson ether synthesis reaction as it involves the treatment of sodium alkoxide with a suitable alkyl halide to form ether. Here we see that cyclopentanol reacts with sodium hydride to form sodium salt of cyclopentolate. After that it reacts with methyl iodide to form an ether named methylcyclopentane.
Williamson Ether Synthesis usually takes place by a \[{S_N}2\] reaction mechanism where we see that a primary alkyl halide reacts with an alkoxide ion. \[{S_N}2\] Pathway is required for the synthesis of this reaction. The alkyl halide is usually primary or secondary. The Ethers delivered in this manner have more carbon ions than both of the beginning materials and accordingly are more perplexing structures.
Therefore, the correct answer is option A.
Note: Alkoxide ions are good nucleophiles and displace halide ions from alkyl halides resulting in the formation of a new carbon oxygen bond. Alkoxide is produced by treatment of alcohols with either a base or an alkali metal. This method is considered the best for the preparation of ethers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




