
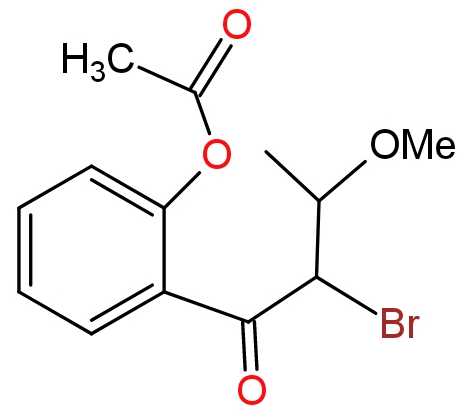
The product of this reaction will be?
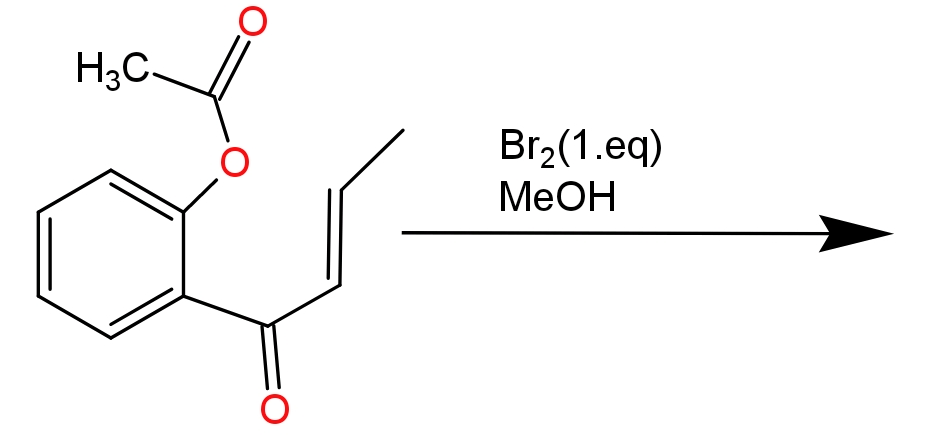
(1)
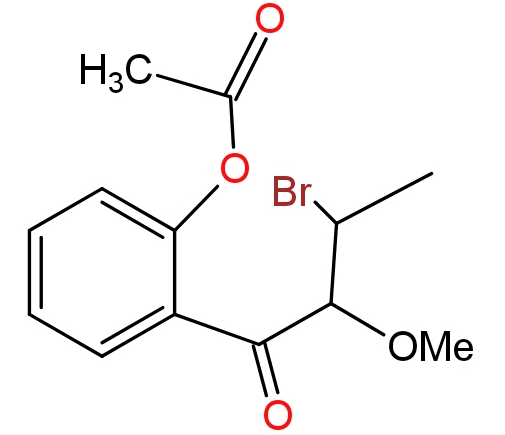
(2)
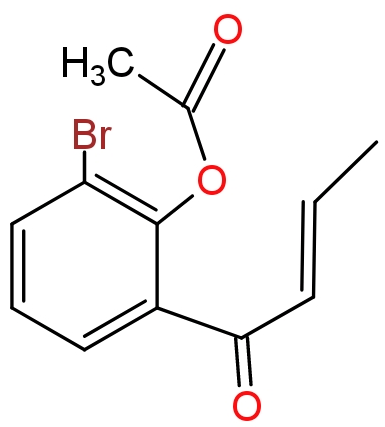
(3)
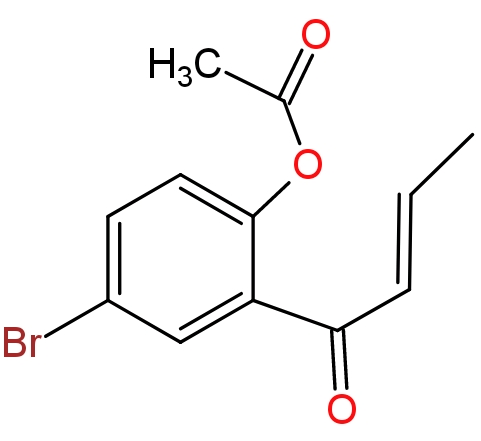
(4)





Answer
572.7k+ views
Hint This reaction is an example of both electrophilic and nucleophilic addition reaction. Alkene is an electron rich centre which mainly shows electrophilic addition reaction.
Complete Step by step solution :
This reaction is completed into two steps - In the first step bromine acts as an electrophile and attacks the double bond of the substrate compound. In the approach of bromine double bond migrates toward the electrophile and electrophilic gets attached to the double bond. Since bromine has lone pairs of electrons, it will donate the lone pair to the other carbon which has positive charge and forms a cyclic halonium ion as an intermediate.
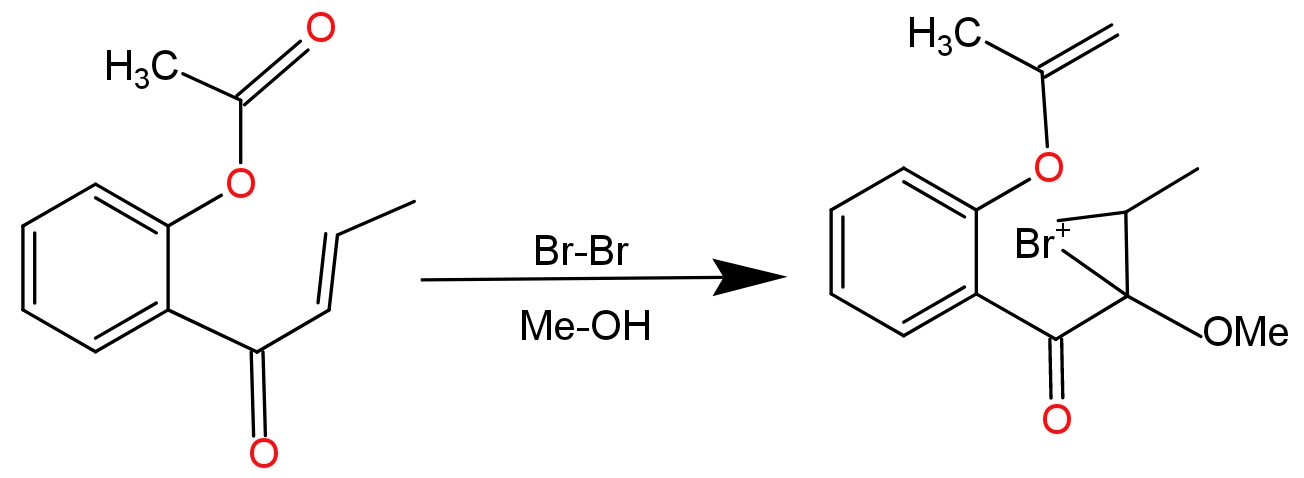
In the second step- methyl alcohol which is present in the form of solvent acts as a nucleophile and attacks the electron deficient carbon atom. Due to formation of cyclic halonium ion anti-addition of methyl alcohol takes place. To compensate the positive charge in oxonium ion it loses its hydrogen atom which will be accepted by bromide ion present in the solution.
Note: Br-Br bond is generally a non- polar bond but when it approaches the alkene(double bond or an electron rich centre) of the complex compound it gets polarised and gets induced charge on the bromine atom which acts as an electrophile.
-Due to the formation of cyclic halonium ions there is no re-arrangement of charge.
-Anti -addition of alcoholic grope takes place because cyclic halonium ion prevents the nucleophile from attacking from the same side.
Complete Step by step solution :
This reaction is completed into two steps - In the first step bromine acts as an electrophile and attacks the double bond of the substrate compound. In the approach of bromine double bond migrates toward the electrophile and electrophilic gets attached to the double bond. Since bromine has lone pairs of electrons, it will donate the lone pair to the other carbon which has positive charge and forms a cyclic halonium ion as an intermediate.

In the second step- methyl alcohol which is present in the form of solvent acts as a nucleophile and attacks the electron deficient carbon atom. Due to formation of cyclic halonium ion anti-addition of methyl alcohol takes place. To compensate the positive charge in oxonium ion it loses its hydrogen atom which will be accepted by bromide ion present in the solution.
Note: Br-Br bond is generally a non- polar bond but when it approaches the alkene(double bond or an electron rich centre) of the complex compound it gets polarised and gets induced charge on the bromine atom which acts as an electrophile.
-Due to the formation of cyclic halonium ions there is no re-arrangement of charge.
-Anti -addition of alcoholic grope takes place because cyclic halonium ion prevents the nucleophile from attacking from the same side.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




