
The product of the following reaction:
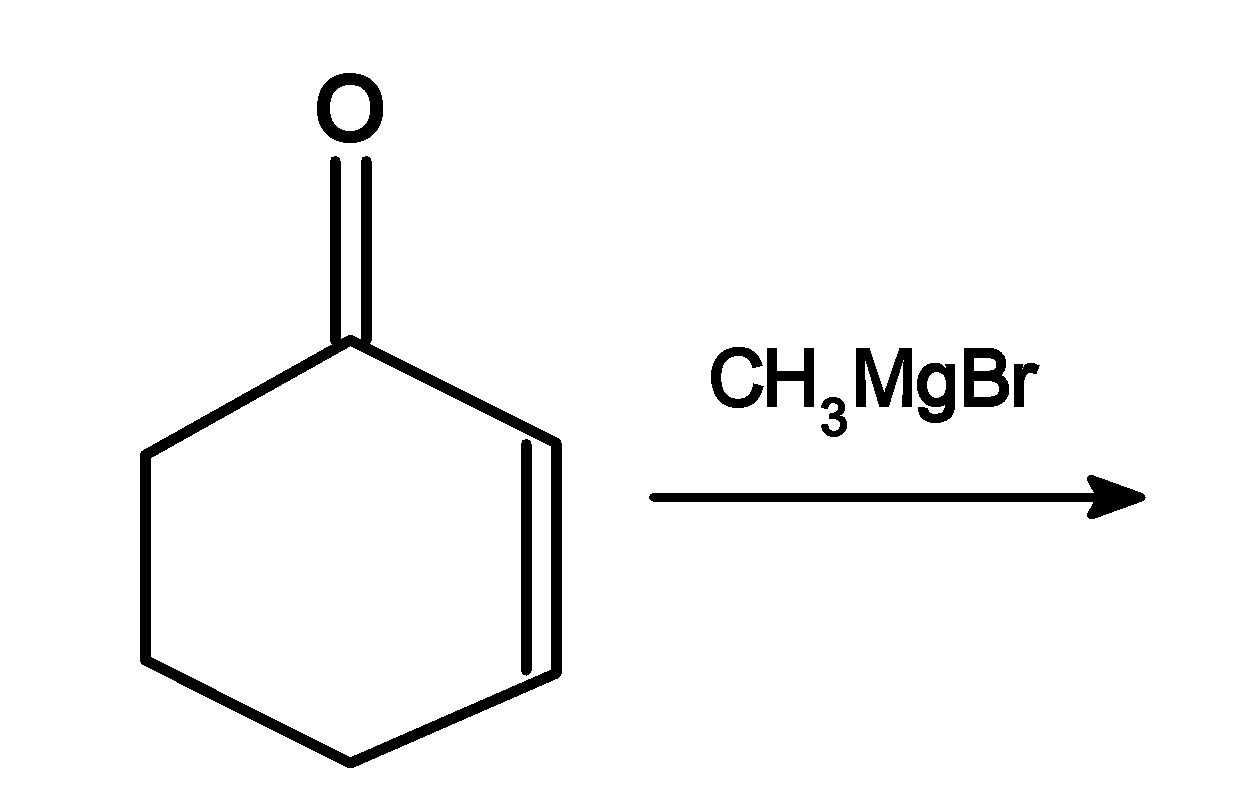

Answer
565.5k+ views
Hint:. The Grignard reagent $\text{ }\left( \text{RMgX} \right)\text{ }$ is a highly polar molecule. The Grignard reagent is a versatile reagent which reacts with aldehyde, ketone, and esters to form addition products where these are decomposed further to give alcohol. The Grignard reagent attacks the carbonyl carbon atom and generates tertiary alcohol.
Complete step by step answer:
- The Grignard reagent $\text{ }\left( \text{RMgX} \right)\text{ }$ is alkyl or aryl magnesium halides. The $\text{ C}-\text{Mg }$ bond in the Grignard reagent $\text{ }\overset{\delta -}{\mathop{\text{R}}}\,-\overset{\delta +}{\mathop{\text{Mg}}}\,-\text{X }$ is highly polar because carbon is electronegative relative to electropositive magnesium. Due to the polar nature of $\text{ C}-\text{Mg }$ the bond, Grignard reagents are very versatile reagents in organic synthesis. The Grignard reagent reacts with aldehyde, ketones, and esters to form addition products which decompose with dil.$\text{ HCl }$ or dil.$\text{ }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$ to give alcohols.
- Here, the carbon double bond shifts on the oxygen atom and generates a positively charged carbon atom. In the next step the methyl group of the Grignard reagent $\text{ C}{{\text{H}}_{\text{3}}}\text{MgBr }$ attacks on the positively charged carbon atom. The methyl group is added to the electron-deficient carbon atom. In the next step water molecules attack on the oxygen atom such that the oxygen atom abstracts a hydrogen atom from the water molecules. This results in the formation of 1-methylcyclohex-2-enol.
The course of reaction is as shown below:
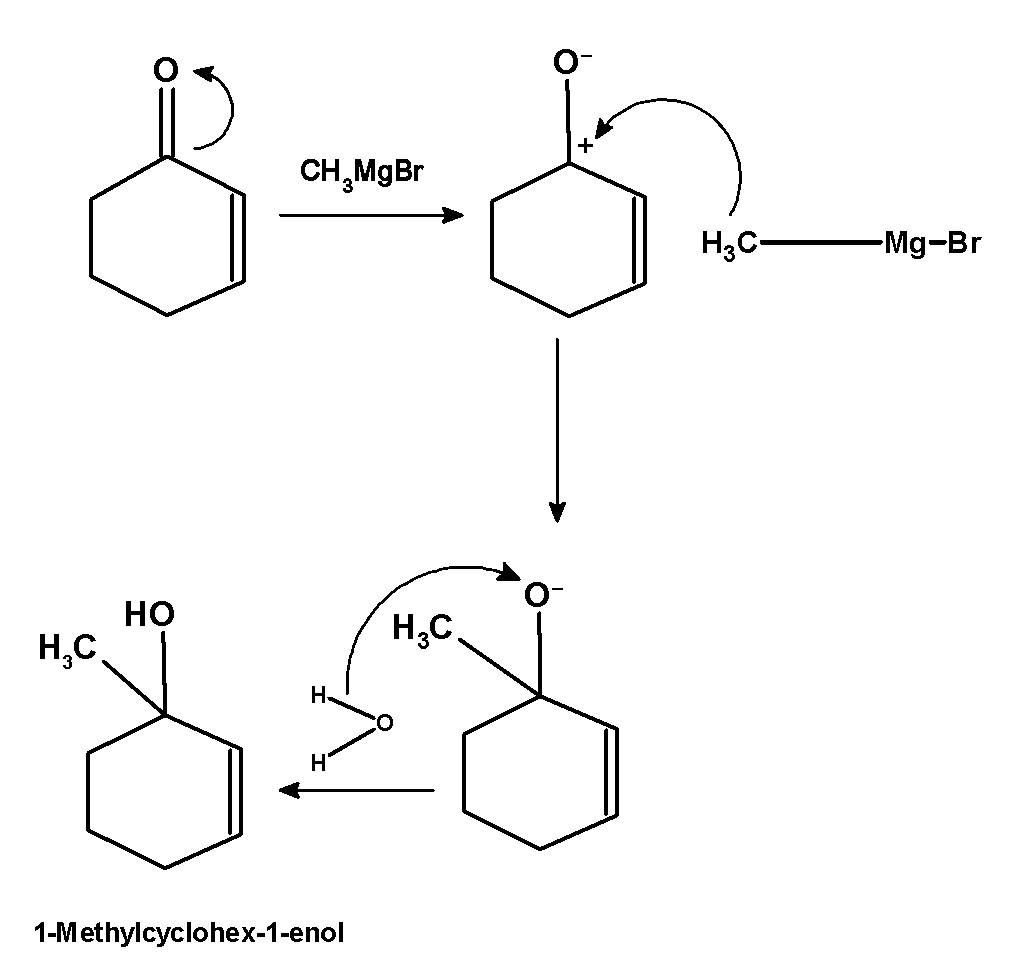
The obtained product is 1-methylcyclohex-1-enol.
Note: Note that Grignard reagent acts as a nucleophile because of the slight negative character of a carbon atom and partial positive character of carbon of carbonyl compound. The attacks take place in such a manner that the ketones produce tertiary alcohol.
Complete step by step answer:
- The Grignard reagent $\text{ }\left( \text{RMgX} \right)\text{ }$ is alkyl or aryl magnesium halides. The $\text{ C}-\text{Mg }$ bond in the Grignard reagent $\text{ }\overset{\delta -}{\mathop{\text{R}}}\,-\overset{\delta +}{\mathop{\text{Mg}}}\,-\text{X }$ is highly polar because carbon is electronegative relative to electropositive magnesium. Due to the polar nature of $\text{ C}-\text{Mg }$ the bond, Grignard reagents are very versatile reagents in organic synthesis. The Grignard reagent reacts with aldehyde, ketones, and esters to form addition products which decompose with dil.$\text{ HCl }$ or dil.$\text{ }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$ to give alcohols.
- Here, the carbon double bond shifts on the oxygen atom and generates a positively charged carbon atom. In the next step the methyl group of the Grignard reagent $\text{ C}{{\text{H}}_{\text{3}}}\text{MgBr }$ attacks on the positively charged carbon atom. The methyl group is added to the electron-deficient carbon atom. In the next step water molecules attack on the oxygen atom such that the oxygen atom abstracts a hydrogen atom from the water molecules. This results in the formation of 1-methylcyclohex-2-enol.
The course of reaction is as shown below:

The obtained product is 1-methylcyclohex-1-enol.
Note: Note that Grignard reagent acts as a nucleophile because of the slight negative character of a carbon atom and partial positive character of carbon of carbonyl compound. The attacks take place in such a manner that the ketones produce tertiary alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




