
The product obtained when benzoyl acetic acid is heated with soda-lime is :
(A)
(B)
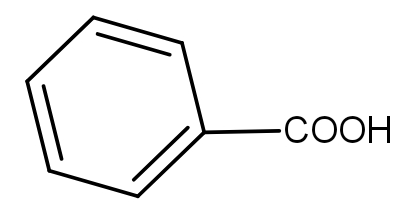
(C)
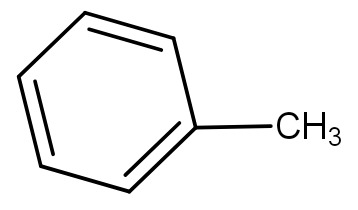
(D)
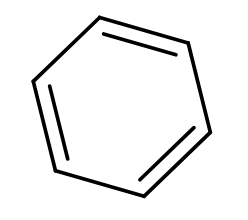




Answer
582.9k+ views
Hint: Soda-lime absorbs carbon dioxide and this reaction is a decarboxylation reaction. As a result, one carbon evolves out in the form of carbon dioxide. So, the product should contain one carbon atom less than the reactant. Further, the product contains a ketone.
Complete step by step answer :
In this type of questions, let us see the structure of reactants and try to guess what type of reactions they could give.
The structure of benzoyl acetic acid is as -
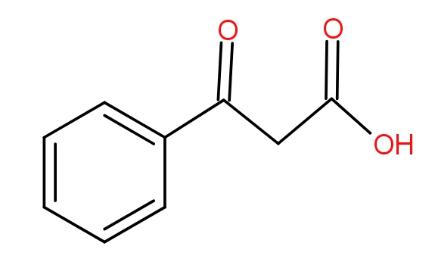
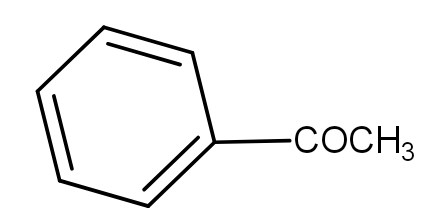
And the soda lime is a mixture of Sodium hydroxide and calcium hydroxide. It absorbs carbon dioxide. When the benzoyl acetic acid is heated with soda-lime, we get acetophenone. The decarboxylation reaction takes place and as a result, the product has one carbon less than the reactant because that carbon formed carbon dioxide by combining with oxygen. The reaction is as -
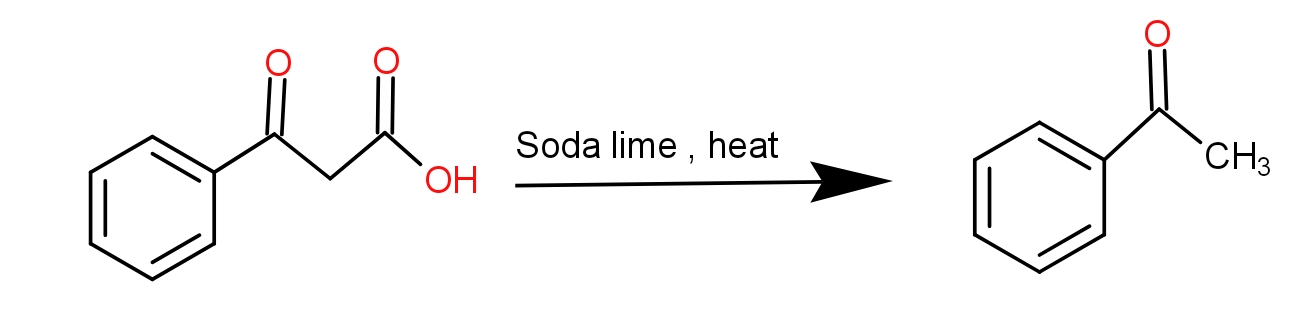
Thus, the acetophenone is the product.
And the option a.) is the correct answer.
Note : Soda-lime is used in submarines, rebreathers, recompression chambers and general anaesthesia to remove carbon dioxide from the breathing gases. It is even used to prevent poisoning by carbon dioxide. It is a greyish-white granular in appearance. During this reaction, the hydrogen of the hydroxyl group is abstracted. As a result, the oxygen acquires negative charge and it tries to stabilise it by resonance. This leads to the formation of carbon dioxide which leaves the reaction and the methylene acquires a hydrogen atom to form the methyl group.
Complete step by step answer :
In this type of questions, let us see the structure of reactants and try to guess what type of reactions they could give.
The structure of benzoyl acetic acid is as -

And the soda lime is a mixture of Sodium hydroxide and calcium hydroxide. It absorbs carbon dioxide. When the benzoyl acetic acid is heated with soda-lime, we get acetophenone. The decarboxylation reaction takes place and as a result, the product has one carbon less than the reactant because that carbon formed carbon dioxide by combining with oxygen. The reaction is as -
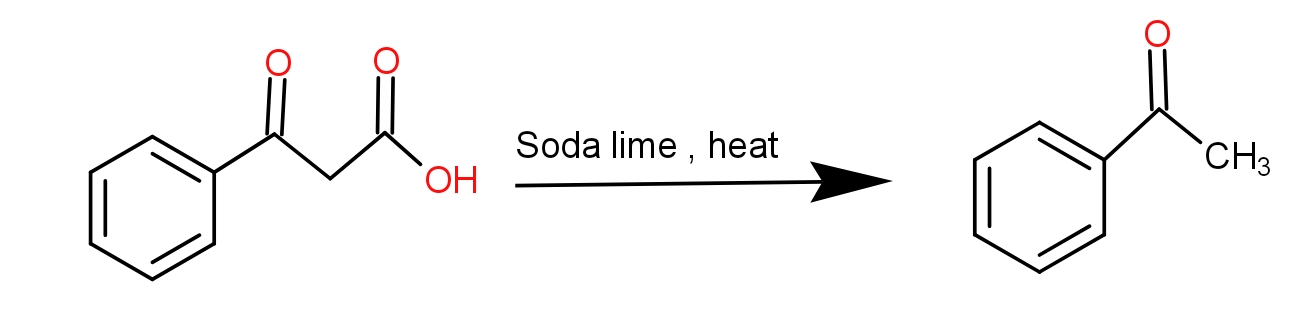
Thus, the acetophenone is the product.
And the option a.) is the correct answer.
Note : Soda-lime is used in submarines, rebreathers, recompression chambers and general anaesthesia to remove carbon dioxide from the breathing gases. It is even used to prevent poisoning by carbon dioxide. It is a greyish-white granular in appearance. During this reaction, the hydrogen of the hydroxyl group is abstracted. As a result, the oxygen acquires negative charge and it tries to stabilise it by resonance. This leads to the formation of carbon dioxide which leaves the reaction and the methylene acquires a hydrogen atom to form the methyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




