

The product A is.

Answer
583.5k+ views
Hint: We know that if we associate the degree of an organic compound with alpha hydrogen in presence of a base to grant an alcohol, it's known as an aldol chemical reaction. Reactions during which a bigger molecule is made from smaller elements, with the elimination of an awfully tiny by-product like water, are termed Condensations.
Complete answer:
We must remember that the product of aldehyde-alcohol reactions frequently experience a consecutive removal of water, created from associate degree alpha-hydrogen and also the beta-hydroxy cluster.
The product of this-elimination reaction is associate degree alpha, beta-unsaturated organic compound or organic compound. Base-catalyzed removal happens with heat. The extra steadiness provided by the conjugated carbonyl system of the merchandise makes some aldehyde-alcohol reactions thermodynamically determined and mixtures of stereoisomers are obtained from some reactions.
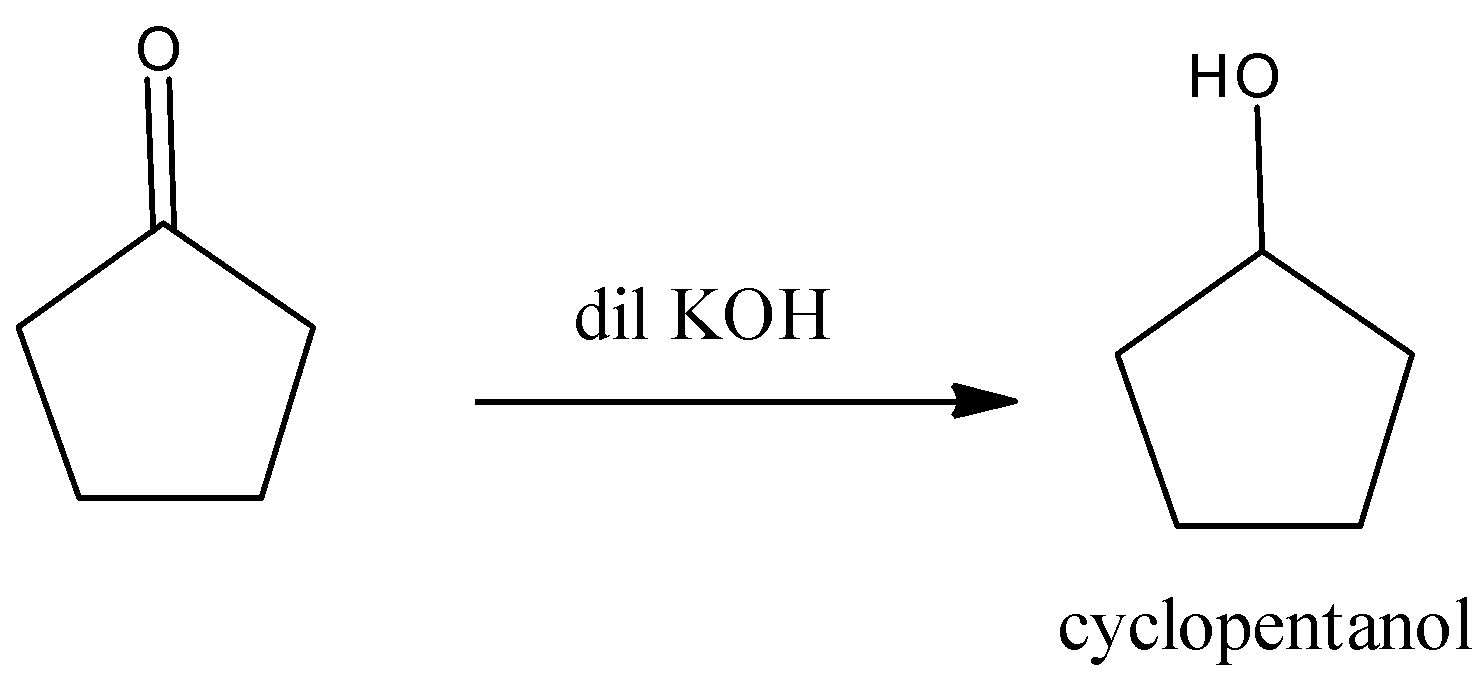
The product A is cyclopentanol.
Additional note:
We have to remember that the aldehydes with alpha element atoms endure deprotonation because of the powerfully basic conditions of the reaction, leading to enolates and/or aldehyde-alcohol reactions of these enolates were beta-hydroxy aldehydes or ketones are obtained. Therefore, it isn't shocking that the reaction produces solely the required alcohol and acid at ideal conditions. This is {often this can be} often why the crossed Cannizzaro reaction is a lot of unremarkably used. A kill organic compound is combined with a lot of valuable chemicals and aldehyde is used as a reducer, oxidizing it to metallic element format. The required alcohol is obtained from the reduction of the alternative organic compound chemical. Since 2 completely different aldehydes are usually fully reborn into the required product, the yield of the dear chemical is inflated.
Crossed Cannizzaro reaction between aldehyde and Benzaldehyde:
\[{C_6}{H_5} - CHO + \left( {Formaldehyde} \right)HCHO\xrightarrow{{NaOH}}HCOOH + {C_6}{H_5} - C{H_2} - OH\]
As there's no lepton donating cluster on aldehyde, the initial nucleophilic addition of hydroxyl ion is quicker on aldehyde because of that aldehyde oxidized simply and forms acid. Thus, the alcohol fashioned is barely radical alcohol and not methyl alcohol.
Note:
We must remember that the chemical change happens once you have got a carbonyl with a nucleon on the adjacent (alpha) carbon - associate degree enolizable alpha nucleon. Below acid or base chemical action, associate degree organic compounds are usually fashioned, which can attack another carbonyl to supply a beta-hydroxy carbonyl compound.
The Cannizzaro reaction will happen once there don't seem to be any enolizable alpha protons. This is often a disproportionation reaction, wherever associate degree organic compound reacts with associate degree other molecule of itself to form an alcohol and an acid.
Complete answer:
We must remember that the product of aldehyde-alcohol reactions frequently experience a consecutive removal of water, created from associate degree alpha-hydrogen and also the beta-hydroxy cluster.

The product of this-elimination reaction is associate degree alpha, beta-unsaturated organic compound or organic compound. Base-catalyzed removal happens with heat. The extra steadiness provided by the conjugated carbonyl system of the merchandise makes some aldehyde-alcohol reactions thermodynamically determined and mixtures of stereoisomers are obtained from some reactions.
The product A is cyclopentanol.
Additional note:
We have to remember that the aldehydes with alpha element atoms endure deprotonation because of the powerfully basic conditions of the reaction, leading to enolates and/or aldehyde-alcohol reactions of these enolates were beta-hydroxy aldehydes or ketones are obtained. Therefore, it isn't shocking that the reaction produces solely the required alcohol and acid at ideal conditions. This is {often this can be} often why the crossed Cannizzaro reaction is a lot of unremarkably used. A kill organic compound is combined with a lot of valuable chemicals and aldehyde is used as a reducer, oxidizing it to metallic element format. The required alcohol is obtained from the reduction of the alternative organic compound chemical. Since 2 completely different aldehydes are usually fully reborn into the required product, the yield of the dear chemical is inflated.
Crossed Cannizzaro reaction between aldehyde and Benzaldehyde:
\[{C_6}{H_5} - CHO + \left( {Formaldehyde} \right)HCHO\xrightarrow{{NaOH}}HCOOH + {C_6}{H_5} - C{H_2} - OH\]
As there's no lepton donating cluster on aldehyde, the initial nucleophilic addition of hydroxyl ion is quicker on aldehyde because of that aldehyde oxidized simply and forms acid. Thus, the alcohol fashioned is barely radical alcohol and not methyl alcohol.
Note:
We must remember that the chemical change happens once you have got a carbonyl with a nucleon on the adjacent (alpha) carbon - associate degree enolizable alpha nucleon. Below acid or base chemical action, associate degree organic compounds are usually fashioned, which can attack another carbonyl to supply a beta-hydroxy carbonyl compound.
The Cannizzaro reaction will happen once there don't seem to be any enolizable alpha protons. This is often a disproportionation reaction, wherever associate degree organic compound reacts with associate degree other molecule of itself to form an alcohol and an acid.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE

How is democracy better than other forms of government class 12 social science CBSE

What are the major means of transport Explain each class 12 social science CBSE




