
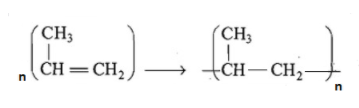
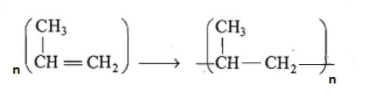
The polymerization of propene to linear polypropene is represented by the reaction where n has large integral value, the average enthalpies of bond dissociation for $(C==C)\text{ and (C-C) }$at 298K are +590 and +331$kJmo{{l}^{-1}}$, respectively. The enthalpy of polymerization is -360$kJmo{{l}^{-1}}$. Find the value of n.
(A) n = 5
(B) n = 2
(C) n = 7
(D) none of these

Answer
583.2k+ views
Hint: Polymerization is the process by which a large number of monomers react together and form a polymer. The macromolecules which are produced by the process of polymerization have a linear or a branched structure depending on the monomer used.
Complete step by step solution:
The bond dissociation energy is an energy which helps to measure the strength of the bond, higher the value of bond dissociation energy higher is the bond strength. Heat of polymerization of a polymer largely depends on the monomer.
Given in the question:
The average bond dissociation enthalpy for $(C==C)$ is = 298 $kJmo{{l}^{-1}}$
The average bond dissociation enthalpy for $\text{ (C-C) }$is = 331 $kJmo{{l}^{-1}}$
The enthalpy of polymerization is = -360$kJmo{{l}^{-1}}$
In the polymerization reaction which is given in the question we can see that breaking of one double bond leads to the formation of two single bond
Enthalpy of polymerization per mole= \[590-2X331\] = -72 $kJmo{{l}^{-1}}$
The given enthalpy of polymerization is = -360$kJmo{{l}^{-1}}$
The value of n will be the ratio of given enthalpy or polymerization to the enthalpy of polymerization per mole
Value of n = $(\dfrac{-360kJmo{{l}^{-1}}}{-72kJmo{{l}^{-1}}})$= 5
Hence the correct option is option (A).
Note: Monomers are a molecule or any of a class of a compound which reacts with other molecules and form polymers or we can say that Polymers are a type of synthetic substances which are made up of simpler units called monomers. Polymer chain can have an unspecified number of monomer units.
Complete step by step solution:
The bond dissociation energy is an energy which helps to measure the strength of the bond, higher the value of bond dissociation energy higher is the bond strength. Heat of polymerization of a polymer largely depends on the monomer.
Given in the question:
The average bond dissociation enthalpy for $(C==C)$ is = 298 $kJmo{{l}^{-1}}$
The average bond dissociation enthalpy for $\text{ (C-C) }$is = 331 $kJmo{{l}^{-1}}$
The enthalpy of polymerization is = -360$kJmo{{l}^{-1}}$
In the polymerization reaction which is given in the question we can see that breaking of one double bond leads to the formation of two single bond

Enthalpy of polymerization per mole= \[590-2X331\] = -72 $kJmo{{l}^{-1}}$
The given enthalpy of polymerization is = -360$kJmo{{l}^{-1}}$
The value of n will be the ratio of given enthalpy or polymerization to the enthalpy of polymerization per mole
Value of n = $(\dfrac{-360kJmo{{l}^{-1}}}{-72kJmo{{l}^{-1}}})$= 5
Hence the correct option is option (A).
Note: Monomers are a molecule or any of a class of a compound which reacts with other molecules and form polymers or we can say that Polymers are a type of synthetic substances which are made up of simpler units called monomers. Polymer chain can have an unspecified number of monomer units.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




