
The oxidation number of Co in the complex ion is:
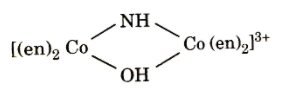
(A) +3
(B) +2
(C) +4
(D) +6

Answer
526k+ views
Hint: To solve this problem we have to consider the oxidation state of cobalt as x and then by adding the oxidation state of the other elements which will be equal to the net charge present in the molecule.
Complete step by step solution:
In the given question we have to calculate the oxidation state of Co in the given complex. So firstly we should know that the given complex is a coordination compound in which the molecules are attached are an en group which is also known as ethylenediamine a chlorine molecule.
Ethylenediamine is a type of bidentate ligand which means that this molecule consists of two donor atoms but it is a neutral atom which means that the oxidation state is 0.
Let the oxidation state of cobalt is x and we know that the oxidation state of NH is -2 and OH is -1 and the total charge is +3 hence the oxidation state of Co is :
\[\begin{align}
& 2(x)+1(-2)+1(-1)=+3 \\
& x=+3 \\
\end{align}\]
Hence, the correct answer is option (A) i.e. the oxidation number of Co in the complex ion is +3.
Note: In the given molecule the ligand which has two donor sites is known as a bidentate ligand. When a ligand has multiple sites which act as a donor atom then it forms the ring-like structure called chelate and the ligand is known as the chelating ligand which forms a stable structure. Oxidation state is the number which is assigned to an element in chemical combination which usually represents the number of electrons which are loosed or gained by that element.
Complete step by step solution:
In the given question we have to calculate the oxidation state of Co in the given complex. So firstly we should know that the given complex is a coordination compound in which the molecules are attached are an en group which is also known as ethylenediamine a chlorine molecule.
Ethylenediamine is a type of bidentate ligand which means that this molecule consists of two donor atoms but it is a neutral atom which means that the oxidation state is 0.
Let the oxidation state of cobalt is x and we know that the oxidation state of NH is -2 and OH is -1 and the total charge is +3 hence the oxidation state of Co is :
\[\begin{align}
& 2(x)+1(-2)+1(-1)=+3 \\
& x=+3 \\
\end{align}\]
Hence, the correct answer is option (A) i.e. the oxidation number of Co in the complex ion is +3.
Note: In the given molecule the ligand which has two donor sites is known as a bidentate ligand. When a ligand has multiple sites which act as a donor atom then it forms the ring-like structure called chelate and the ligand is known as the chelating ligand which forms a stable structure. Oxidation state is the number which is assigned to an element in chemical combination which usually represents the number of electrons which are loosed or gained by that element.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




