
The most acidic compound among the following is:
A) Phenol
B) Ethanol
C) $3,5 - $ dinitrophenol
D) $4,4 - $ methoxyphenol
Answer
553.8k+ views
Hint: To determine the most acidic compound we will have to see that if an electron withdrawing group is present or not. The electron withdrawing group makes a phenol more acidic as it will stabilize the phenoxide ion by the delocalization of the negative charge and also due to the inductive effect.
Complete answer:
We know that phenols will be more acidic than ethanol because the phenols after losing a proton will form a phenoxide ion. This negative charge present on the phenoxide ion will undergo resonance and is therefore very stable.
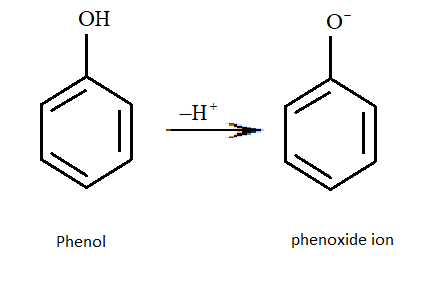
But here we need to see which of the following three types of phenols is most acidic.
So, we know that we determine the strength of an acid by the presence of electron withdrawing groups and also the position at which the groups are attached to the phenol.
Nitro group is an electron withdrawing group and shows both the effects i.e., the M-effect (mesomeric effect) also known as resonance and the I-effect or the inductive effect. The resonance effect causes the nitro group to withdraw electrons from the ortho and para positions. By inductive effect also it withdraws electrons but this effect is weaker than the resonance effect.
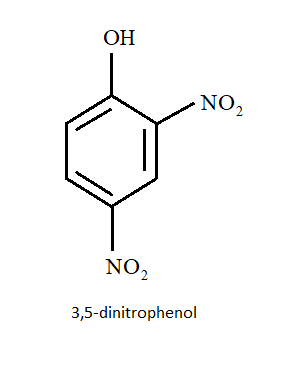
Nitro group by negative inductive effect withdraws electrons in the following order:
$Ortho > meta > para$
Hence, the correct answer option is (D) i.e. Hydrogen gas.
Note: Ortho nitrophenol is less acidic than para nitrophenol because of the intermolecular hydrogen bonding which makes the loss of proton very difficult. So, para nitrophenol is more acidic. On the other hand, the nitro group on increasing the acidity of phenol will decrease its basicity which means that nitrogen present in the nitro group will become less basic.
Complete answer:
We know that phenols will be more acidic than ethanol because the phenols after losing a proton will form a phenoxide ion. This negative charge present on the phenoxide ion will undergo resonance and is therefore very stable.

But here we need to see which of the following three types of phenols is most acidic.
So, we know that we determine the strength of an acid by the presence of electron withdrawing groups and also the position at which the groups are attached to the phenol.
Nitro group is an electron withdrawing group and shows both the effects i.e., the M-effect (mesomeric effect) also known as resonance and the I-effect or the inductive effect. The resonance effect causes the nitro group to withdraw electrons from the ortho and para positions. By inductive effect also it withdraws electrons but this effect is weaker than the resonance effect.

Nitro group by negative inductive effect withdraws electrons in the following order:
$Ortho > meta > para$
Hence, the correct answer option is (D) i.e. Hydrogen gas.
Note: Ortho nitrophenol is less acidic than para nitrophenol because of the intermolecular hydrogen bonding which makes the loss of proton very difficult. So, para nitrophenol is more acidic. On the other hand, the nitro group on increasing the acidity of phenol will decrease its basicity which means that nitrogen present in the nitro group will become less basic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




