
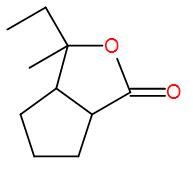
The major product for the following reaction is:
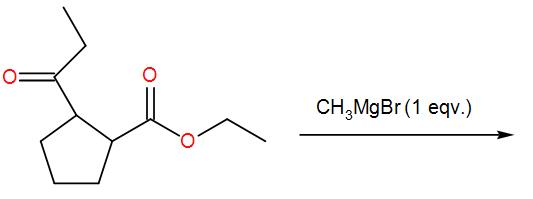
(A)
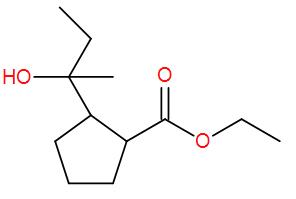
(B)
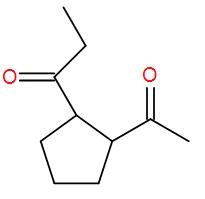
(C)
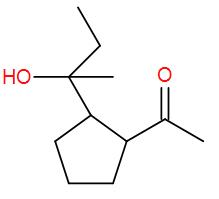
(D)





Answer
573.6k+ views
HINT: To answer this, you need to notice that a Grignard reagent is given as a reagent. In it the alkyl group bears a partial negative charge. Remember that 1 equivalent of Grignard reacts with carbonyls to give alcohols. You can answer the question using this.
Complete step by step solution:
In the given reaction, the reagent given to us is a Grignard reagent. To answer this, firstly let’s discuss what it is and how it works.
This reagent is magnesium containing a chemical compound with the formula- R-Mg-X. R can be any alkyl group and X stands for halide. The most common example is $C{{H}_{3}}MgCl$. It is partly ionic $\left( \overset{\delta -}{\mathop{R}}\,\cdots \overset{\delta +}{\mathop{Mg}}\,X \right)$ and here the alkyl group bears a partial negative charge. It acts as a strong base and thus reacts with acidic hydrogen to give us alkanes.
Here, the reactant given to us is a five-membered ring with carbonyl carbon. So, let’s discuss how Grignard reagent reacts with carbonyl groups.
The carbonyl group of aldehydes or ketones with Grignard reagents gives us alcohol. Whether the alcohol will be primary, secondary or tertiary depends upon the nature of the alkyl group. Now let us see the given question.
Here, we have an ester but esters add to 2 equivalents of Grignard reagent and here we have 1 equivalent. So, we can write down the reaction mechanism as-
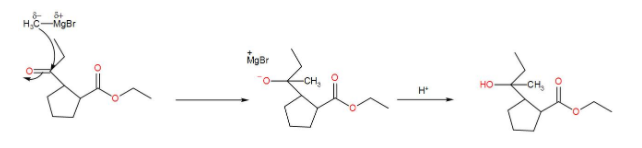
Therefore, the correct answer is option-(A).
NOTE: Grignard reagent is an organometallic reagent. It is used in various reactions and synthetically used to form new C – C bonds as we have already seen in the above reaction. For esters, two equivalents of Grignard is required because after the first equivalent reacts, it results in the formation of a ketone which can undergo further reaction to give an alcohol and thus using up the second equivalent of the reagent. Whereas in aldehydes and ketones, there is no reacting site left to react once the alcohol is already formed.
Complete step by step solution:
In the given reaction, the reagent given to us is a Grignard reagent. To answer this, firstly let’s discuss what it is and how it works.
This reagent is magnesium containing a chemical compound with the formula- R-Mg-X. R can be any alkyl group and X stands for halide. The most common example is $C{{H}_{3}}MgCl$. It is partly ionic $\left( \overset{\delta -}{\mathop{R}}\,\cdots \overset{\delta +}{\mathop{Mg}}\,X \right)$ and here the alkyl group bears a partial negative charge. It acts as a strong base and thus reacts with acidic hydrogen to give us alkanes.
Here, the reactant given to us is a five-membered ring with carbonyl carbon. So, let’s discuss how Grignard reagent reacts with carbonyl groups.
The carbonyl group of aldehydes or ketones with Grignard reagents gives us alcohol. Whether the alcohol will be primary, secondary or tertiary depends upon the nature of the alkyl group. Now let us see the given question.
Here, we have an ester but esters add to 2 equivalents of Grignard reagent and here we have 1 equivalent. So, we can write down the reaction mechanism as-

Therefore, the correct answer is option-(A).
NOTE: Grignard reagent is an organometallic reagent. It is used in various reactions and synthetically used to form new C – C bonds as we have already seen in the above reaction. For esters, two equivalents of Grignard is required because after the first equivalent reacts, it results in the formation of a ketone which can undergo further reaction to give an alcohol and thus using up the second equivalent of the reagent. Whereas in aldehydes and ketones, there is no reacting site left to react once the alcohol is already formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




