
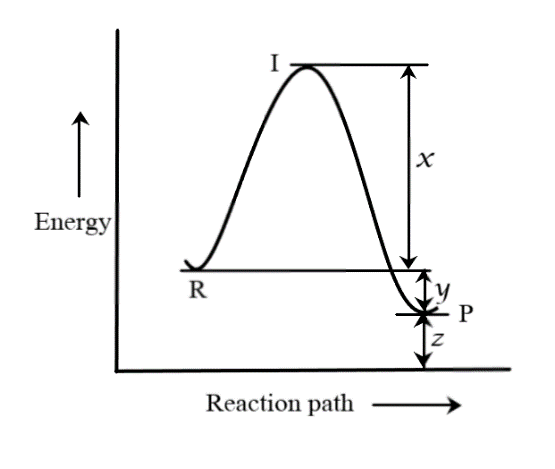
The energy profile diagram for the reaction, $CO(g) + N{O_2}(g) \rightleftharpoons C{O_2}(g) + NO(g)$ is given in the figure, the activation energy of the backward reaction is:
A. $x$
B. $y$
C. $x + y$
D. $x - y$

Answer
573.6k+ views
Hint:Molecules will only react to form a product when they are raised to a certain threshold energy, which is identified as the peak in an energy diagram. Thus, for the backward reaction, the portion of the graph which shows the energy of the final product to the peak will give us the activation energy of the backward reaction.
Complete answer:
The initial energy of the reactant molecules is marked as the point $R$ in the figure. Thus, the initial energy of the reactant molecules, from the figure, is $y + z$.
They are then being raised to a higher energy state. The peak of the graph corresponds to the threshold energy value. The reaction occurs only when the reactants acquire this threshold energy value. The extra energy supplied to the reactants is therefore known as the activation energy for the forward reaction. As we can clearly see, this corresponds to the portion between points $R$ and $I$ in the graph. Hence, activation energy of forward reaction is $x$.
After the reaction, the products are formed at a lower energy level than the reactants, indicated by the point $P$. The difference in energies of the reactants and products is what $y$ denotes. For the backward reaction to happen, the energy of the products must be raised to the same threshold energy, since the threshold energy value is the same for forward and backward reactions. Thus, it should reach the peak from point $P$. Clearly, from the graph, this is denoted by $x + y$.
Hence, the activation energy of the backward reaction is $x + y$ and thus, the correct option to be marked is C.
Note:
The activation energy is generally acquired by supplying heat, that is, increasing the temperature of the reaction conditions. In this case, since the energy level of the products is lower than that of the reactants, heat is being released, and thus, it is an exothermic reaction. Note that catalysts are used to provide an alternate reaction pathway with a lower activation energy, hence, increasing the rate of the reaction.
Complete answer:
The initial energy of the reactant molecules is marked as the point $R$ in the figure. Thus, the initial energy of the reactant molecules, from the figure, is $y + z$.
They are then being raised to a higher energy state. The peak of the graph corresponds to the threshold energy value. The reaction occurs only when the reactants acquire this threshold energy value. The extra energy supplied to the reactants is therefore known as the activation energy for the forward reaction. As we can clearly see, this corresponds to the portion between points $R$ and $I$ in the graph. Hence, activation energy of forward reaction is $x$.
After the reaction, the products are formed at a lower energy level than the reactants, indicated by the point $P$. The difference in energies of the reactants and products is what $y$ denotes. For the backward reaction to happen, the energy of the products must be raised to the same threshold energy, since the threshold energy value is the same for forward and backward reactions. Thus, it should reach the peak from point $P$. Clearly, from the graph, this is denoted by $x + y$.
Hence, the activation energy of the backward reaction is $x + y$ and thus, the correct option to be marked is C.
Note:
The activation energy is generally acquired by supplying heat, that is, increasing the temperature of the reaction conditions. In this case, since the energy level of the products is lower than that of the reactants, heat is being released, and thus, it is an exothermic reaction. Note that catalysts are used to provide an alternate reaction pathway with a lower activation energy, hence, increasing the rate of the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




