
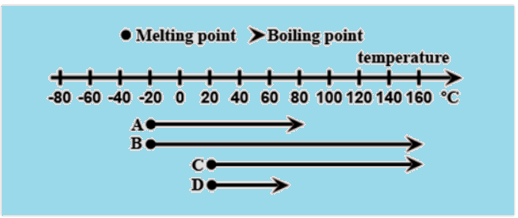
The diagram shows the melting and boiling points of four different substances. Which substance has a melting point at \[ - {20^ \circ }C\] and a boiling point at \[{160^ \circ }C\] ? Also identify the two substances having the same boiling points.
Answer
232.8k+ views
Hint: In this question, we can find the melting point and boiling point of each substance separately. After that we can find the substance for the required melting point and boiling point. It is possible that more than one substance has the same melting point or same boiling point.
Complete step by step solution:
Let us make a table for melting points of the substances and the boiling points of the substances according to the diagram.
According to the above table,
Substance A and B have the melting point \[ - {20^ \circ }C\]. Substance B and C have the boiling point \[{160^ \circ }C\].
So, substance B has the melting point \[ - {20^ \circ }C\] and boiling point \[{160^ \circ }C\].
Now, again from the above table,
Substance B and C have the boiling point \[{160^ \circ }C\].
Additional Information:
Temperature- Temperature can be defined as a physical quantity that expresses hot or cold. We can differentiate an object whether it is cold or hot by temperature.
Melting Point- It is defined as a temperature where the solid phase and liquid phase are in equilibrium.
Boiling Point- It is defined as a temperature at which the vapour pressure of a liquid is equal to the external pressure i.e. surroundings pressure.
Note: It is possible that two substances may have the same melting point and same boiling point. It is also possible that two substances may have only the same melting point. It is also possible that two substances may have only the same boiling point.
Complete step by step solution:
Let us make a table for melting points of the substances and the boiling points of the substances according to the diagram.
| Substance | Melting point$^ \circ C$ | Boiling point$^ \circ C$ |
| A | $ - 20$ | $80$ |
| B | $ - 20$ | \[160\] |
| C | \[20\] | \[160\] |
| D | \[20\] | $70$ |
According to the above table,
Substance A and B have the melting point \[ - {20^ \circ }C\]. Substance B and C have the boiling point \[{160^ \circ }C\].
So, substance B has the melting point \[ - {20^ \circ }C\] and boiling point \[{160^ \circ }C\].
Now, again from the above table,
Substance B and C have the boiling point \[{160^ \circ }C\].
Additional Information:
Temperature- Temperature can be defined as a physical quantity that expresses hot or cold. We can differentiate an object whether it is cold or hot by temperature.
Melting Point- It is defined as a temperature where the solid phase and liquid phase are in equilibrium.
Boiling Point- It is defined as a temperature at which the vapour pressure of a liquid is equal to the external pressure i.e. surroundings pressure.
Note: It is possible that two substances may have the same melting point and same boiling point. It is also possible that two substances may have only the same melting point. It is also possible that two substances may have only the same boiling point.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




