
The diagram below shows four beakers of water P, Q, R and S at different temperature and volume. Which one of the following statements is true?
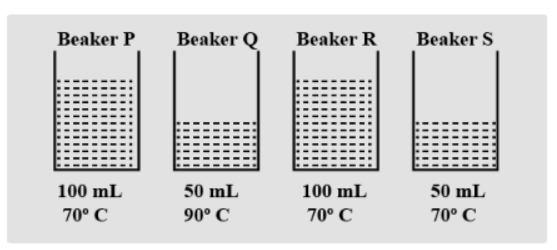
A. Beaker P has the most heat energy
B. Beaker R has more heat energy than beaker Q
C. Beaker S has more heat energy than beaker R
D. Beaker P and beaker S have the same amount of heat energy

Answer
572.4k+ views
Hint: Here we have to apply the concepts of heat energy and internal energy and work. We also have to see how the heat energy is related to temperature and volume.
Step by step answer: Heat energy is the product of the passage of tiny particles in solids, liquids and gases called atoms, molecules or ions. Heat energy may be moved from one particle to another. Move or flow is called heat due to the change in temperature between two objects.
An internal energy intrinsic in each system is the inner energy, which emerges from the molecular condition of movement of matter. The symbol $U$ is utilized for the inner energy and the unit of estimation is the joules.
Internal energy increments with rising temperature and with changes of state or stage from solid to liquid and liquid to gas. Planetary bodies can be thought of as a mixture of heat reservoirs and heat engines. The heat reservoirs store inside energy $E$ , and the heat devices convert a portion of this warm energy into different kinds of mechanical, electrical and chemical energies.
The internal energy is equivalent to the heat of the system. The encompassing heat increments, so the heat of the system diminishes in light of the fact that heat is neither created nor destroyed.
The heat energy present in the measuring glass relies on
-The measure of water present in it, and
-The temperature of the water. As the most measure of water at the most elevated temperature is available in beaker R, hence container R, will have the highest measure of heat energy.
Hence, option B is correct.
Note: Here we have to see which beaker has the highest temperature. Also we have to notice which beaker has the highest water level. It should be kept in mind that here we are talking about the heat content, so mass will be in consideration.
Step by step answer: Heat energy is the product of the passage of tiny particles in solids, liquids and gases called atoms, molecules or ions. Heat energy may be moved from one particle to another. Move or flow is called heat due to the change in temperature between two objects.
An internal energy intrinsic in each system is the inner energy, which emerges from the molecular condition of movement of matter. The symbol $U$ is utilized for the inner energy and the unit of estimation is the joules.
Internal energy increments with rising temperature and with changes of state or stage from solid to liquid and liquid to gas. Planetary bodies can be thought of as a mixture of heat reservoirs and heat engines. The heat reservoirs store inside energy $E$ , and the heat devices convert a portion of this warm energy into different kinds of mechanical, electrical and chemical energies.
The internal energy is equivalent to the heat of the system. The encompassing heat increments, so the heat of the system diminishes in light of the fact that heat is neither created nor destroyed.
The heat energy present in the measuring glass relies on
-The measure of water present in it, and
-The temperature of the water. As the most measure of water at the most elevated temperature is available in beaker R, hence container R, will have the highest measure of heat energy.
Hence, option B is correct.
Note: Here we have to see which beaker has the highest temperature. Also we have to notice which beaker has the highest water level. It should be kept in mind that here we are talking about the heat content, so mass will be in consideration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




