
The coordination number in HCP is:
A. 6
B. 12
C. 18
D. 24
Answer
579.6k+ views
Hint: The coordination number of an atom in a crystal can be defined as the number of atoms in contact with a particular atom in a unit cell. HCP means hexagonal close packed. The repeating unit in a crystal structure is called a unit cell.
Complete step by step answer:
- In the question it is given that we have to find the coordination number in HCP.
- There are 14 types of unit cells found in nature they are called Bravais lattices.
- However in nature most of the unit cells have BCC or FCC or HCP structures in their crystal structure.
- The structure of HCP is as follows.
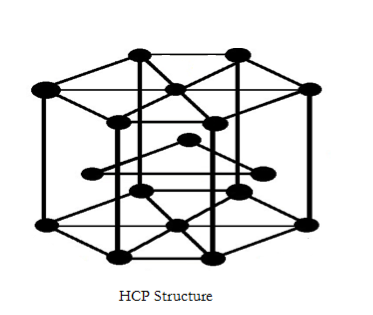
- In the above structure each atom located in the empty space is formed by three adjacent atoms from the top layer, three adjacent atoms from the bottom layer and surrounded by 6 neighboring atoms.
- Therefore there are 12 atoms in touch or coordination with each atom in a Hexagonal close packed structure, then the coordination number of HCP is 12.
- So, the correct option is B.
Note: Don’t be confused with coordination number and number atoms in a unit cell. Both are not the same. The total number of atoms per unit cell in hexagonal close packed is six but the coordination of hexagonal close packed is 12. The number of atoms in FCC unit cells is 4 and the number of atoms in BCC unit cells is 2.
Complete step by step answer:
- In the question it is given that we have to find the coordination number in HCP.
- There are 14 types of unit cells found in nature they are called Bravais lattices.
- However in nature most of the unit cells have BCC or FCC or HCP structures in their crystal structure.
- The structure of HCP is as follows.

- In the above structure each atom located in the empty space is formed by three adjacent atoms from the top layer, three adjacent atoms from the bottom layer and surrounded by 6 neighboring atoms.
- Therefore there are 12 atoms in touch or coordination with each atom in a Hexagonal close packed structure, then the coordination number of HCP is 12.
- So, the correct option is B.
Note: Don’t be confused with coordination number and number atoms in a unit cell. Both are not the same. The total number of atoms per unit cell in hexagonal close packed is six but the coordination of hexagonal close packed is 12. The number of atoms in FCC unit cells is 4 and the number of atoms in BCC unit cells is 2.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




