
The conversion of m-dinitrobenzene into m-nitroaniline can be brought about with :
a.) Sn / HCl
b.) Zn /$N{H_4}Cl$
c.) ${(N{H_4})_2}S$
d.) Zn + Alc. KOH
Answer
590.4k+ views
Hint: The correct answer for this from the options given is the one containing the sulphur atom. All these reagents are reducing reagents and can reduce nitro to amine but the one that reduces selectively is one that contains N and S atoms.
Complete step by step answer :
First, let us see what is happening in the reaction.
In short, m-dinitrobenzene → m-nitroaniline. Structurally, we can say
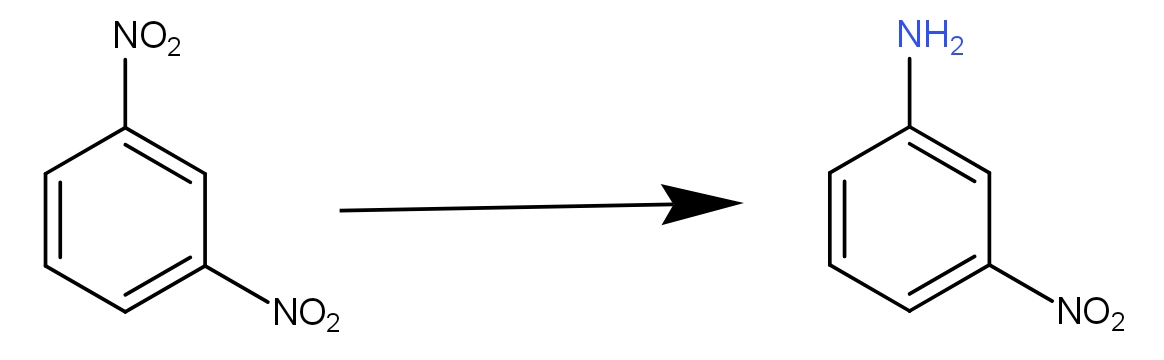
What we see from the above structures is that one of the nitro groups has been replaced with the amino group. So, what exactly has taken place is the reduction. The reduction reaction may be defined as the reaction involving the addition of hydrogen or loss of oxygen if we talk in terms of hydrogen and oxygen. In terms of electrons, it is a different concept. The next thing to observe is that only one nitro group has been replaced and not both. This means there is a reduction at one nitro group only. So, the reduction is selective in nature. Thus, there must be some special conditions involved. When we see all the options given to us, all these are reducing agents. So, all these can reduce the nitrobenzene to aniline. But of all these, the ammonium sulphide selectively reduces one nitro group when two nitro groups are present at meta-position to each other. The reaction to this can be written as -
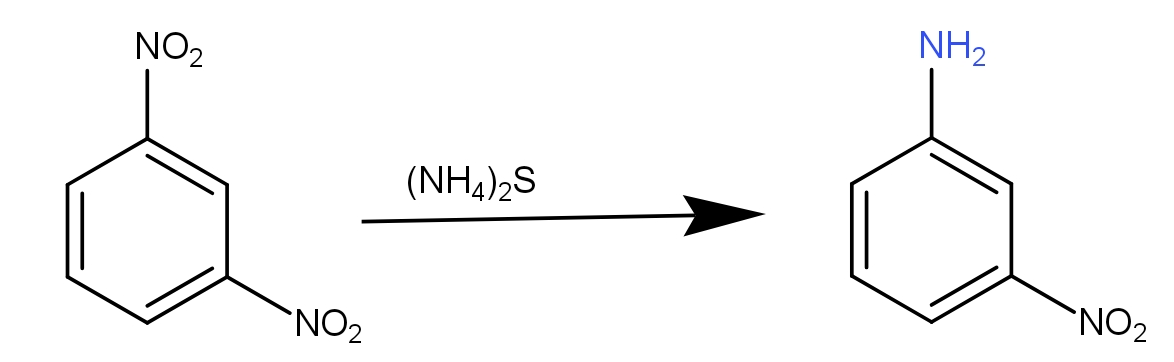
Thus, the option c.) with ammonium sulphide (${(N{H_4})_2}S$) is the correct answer.
Note: It must be noted that Sn / HCl is known as the best reagent for reduction of m- dinitrobenzene to m- nitroaniline but here it can not be used because its use will reduce both the nitro groups to amino groups. Even the zinc with ammonium chloride will reduce both the nitro groups but here we need a reagent which selectively reduces one group.
Complete step by step answer :
First, let us see what is happening in the reaction.
In short, m-dinitrobenzene → m-nitroaniline. Structurally, we can say

What we see from the above structures is that one of the nitro groups has been replaced with the amino group. So, what exactly has taken place is the reduction. The reduction reaction may be defined as the reaction involving the addition of hydrogen or loss of oxygen if we talk in terms of hydrogen and oxygen. In terms of electrons, it is a different concept. The next thing to observe is that only one nitro group has been replaced and not both. This means there is a reduction at one nitro group only. So, the reduction is selective in nature. Thus, there must be some special conditions involved. When we see all the options given to us, all these are reducing agents. So, all these can reduce the nitrobenzene to aniline. But of all these, the ammonium sulphide selectively reduces one nitro group when two nitro groups are present at meta-position to each other. The reaction to this can be written as -

Thus, the option c.) with ammonium sulphide (${(N{H_4})_2}S$) is the correct answer.
Note: It must be noted that Sn / HCl is known as the best reagent for reduction of m- dinitrobenzene to m- nitroaniline but here it can not be used because its use will reduce both the nitro groups to amino groups. Even the zinc with ammonium chloride will reduce both the nitro groups but here we need a reagent which selectively reduces one group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




