
The condensation polymer among the following is:
A.Rubber
B.Protein
C.Poly vinyl chloride
D.Polyethene
Answer
592.5k+ views
Hint: Polymer is a substance consisting of very large molecules, or macromolecules, composed of many repeating units. Due to their broad spectrum of properties, polymers play an important role in everyday life.
Complete step by step answer:
Condensation polymers are the polymers made up from condensation polymerization or we can say the polymerization in which monomers come together to form a larger structural unit. Small molecules are released as by products such as water, methanol, etc.
As we all know that proteins are formed from the amino acids, they form covalent bonds with each other and are linked through peptide linkage in a chain. During the formation of proteins water molecules are released as a byproduct and the whole process works as condensation polymerization.
So protein is a condensation polymer.
An Additional polymer is the polymer which is formed from the simple addition of monomer units without releasing any co-products with it. Rubber, PVC and polyethylene are the examples of additional polymers as they are formed from a simple combination of monomer units.
Preparation reaction of protein:
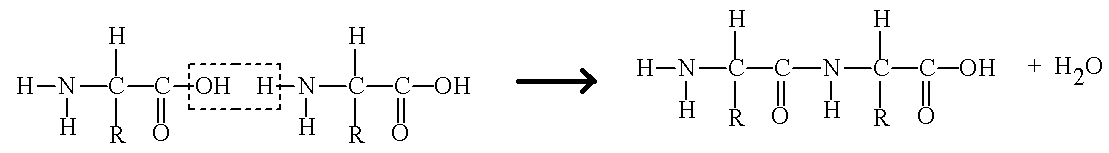
Note:
Condensation polymerization and addition polymerization are different from one another. In both the cases monomer units combine but in condensation polymerization by products like water, methanol, etc. are released but in addition polymerization no by products are seen.
Complete step by step answer:
Condensation polymers are the polymers made up from condensation polymerization or we can say the polymerization in which monomers come together to form a larger structural unit. Small molecules are released as by products such as water, methanol, etc.
As we all know that proteins are formed from the amino acids, they form covalent bonds with each other and are linked through peptide linkage in a chain. During the formation of proteins water molecules are released as a byproduct and the whole process works as condensation polymerization.
So protein is a condensation polymer.
An Additional polymer is the polymer which is formed from the simple addition of monomer units without releasing any co-products with it. Rubber, PVC and polyethylene are the examples of additional polymers as they are formed from a simple combination of monomer units.
Preparation reaction of protein:
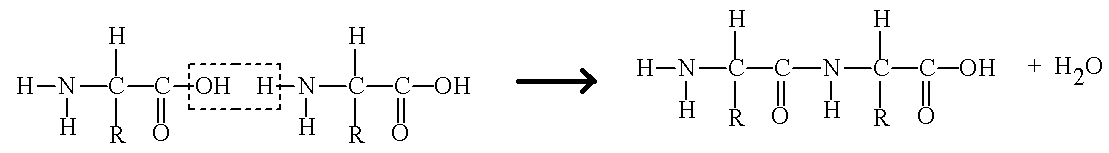
Note:
Condensation polymerization and addition polymerization are different from one another. In both the cases monomer units combine but in condensation polymerization by products like water, methanol, etc. are released but in addition polymerization no by products are seen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




