
The basicity of aniline is weaker in comparison to that of methylamine due to:
A.Hyper conjugative effect of $Me - $ group $MeN{H_2}$
B.Resonance effect of the phenyl group in aniline
C.The lower molecular weight of methylamine compared to that of aniline
D.Resonance effect of $ - N{H_2}$ group in $MeN{H_2}$
Answer
583.2k+ views
Hint: To answer this question, recall the concept of basic strength. Basic strength is defined as the ability of a base to donate the electrons. It depends on the availability of lone pairs of the donor atom.
Complete step by step answer:
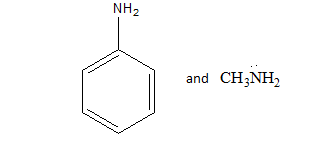
We know that the resonance effect is more effective than inductive effect. We can define the resonance effect as The resonance effect can be defined as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
In aniline resonance effect is present due to conjugation between benzene ring and lone pair present on the amine group which makes the lone pair less available for donation while acting as a base, while in $MeN{H_2}$ only inductive effect is present due to the methyl group due to which lone pair is localized and available for donation. Hence , aniline is less basic than methylamine due to Resonance effect of the phenyl group in aniline.
Thus, the correct option is option B.
Note:
Arrhenius Acid: Hydronium breaks up to yield an hydronium in solution. Arrhenius Base: Hydroxide is dissolved in water as $O{H^ - }$. Bronsted-Lowry Acid: Hydronium is an \[{H^ + }\] donor regardless of solution. Bronsted-Lowry Base: Hydroxide attacks and accepts the \[{H^ + }\] from hydronium. Lewis Acid: The \[{H^ + }\] on Hydronium accepts the attacking electron pair to form a bond. Lewis Base: Hydroxide donates electron pairs present over it to form a bond between itself and \[{H^ + }\].
Complete step by step answer:

We know that the resonance effect is more effective than inductive effect. We can define the resonance effect as The resonance effect can be defined as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
In aniline resonance effect is present due to conjugation between benzene ring and lone pair present on the amine group which makes the lone pair less available for donation while acting as a base, while in $MeN{H_2}$ only inductive effect is present due to the methyl group due to which lone pair is localized and available for donation. Hence , aniline is less basic than methylamine due to Resonance effect of the phenyl group in aniline.
Thus, the correct option is option B.
Note:
Arrhenius Acid: Hydronium breaks up to yield an hydronium in solution. Arrhenius Base: Hydroxide is dissolved in water as $O{H^ - }$. Bronsted-Lowry Acid: Hydronium is an \[{H^ + }\] donor regardless of solution. Bronsted-Lowry Base: Hydroxide attacks and accepts the \[{H^ + }\] from hydronium. Lewis Acid: The \[{H^ + }\] on Hydronium accepts the attacking electron pair to form a bond. Lewis Base: Hydroxide donates electron pairs present over it to form a bond between itself and \[{H^ + }\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




