
Taking an example of an ambidentate ligand, explain why it is called an ambidentate ligand.
Answer
599.7k+ views
Hint: We can think about the word ambidentate grammatically and interpret. We know that ligands donate their electron pair and form coordinate covalent bonds. Denticity of ligand is the number of pairs of electrons donated by the ligand.
Complete step by step answer:
We know that d block elements form coordination compounds as they have empty orbitals to accept electrons from other compounds or elements. Ligands donate and share pairs of electrons metal atoms or ions and form coordination compounds. Number of pairs of electrons donated by ligands is called denticity.
Ambidentate ligand is a ligand which has more than one atom to donate an electron pair but can donate only one pair of electrons. Ambidentate ligands are monodentate ligands with variable donor atoms.
Some ambidentate ligands are $CN^{ - },\quad N{ O }_{ 2 }^{ - },\quad CN{ S }^{ - }$.
Let us consider $CN^{ - }$ ion. Cyanide ion acts as a ligand because of the presence of lone pairs of electrons on both nitrogen and carbon atoms.
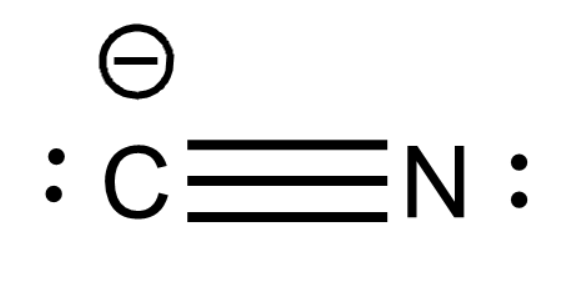
Due to negative charge both carbon and nitrogen atoms have lone pairs of electrons on each of them. And both are capable of donating their lone pair of electrons. But both atoms do not donate their electrons simultaneously.
Note: Though ambidentate ligands have multiple electron donors they cannot form more than one bond because there will be more electron repulsions as 2 electron clouds are very close. And also cyclic structures with small numbered centers are highly unstable.
Complete step by step answer:
We know that d block elements form coordination compounds as they have empty orbitals to accept electrons from other compounds or elements. Ligands donate and share pairs of electrons metal atoms or ions and form coordination compounds. Number of pairs of electrons donated by ligands is called denticity.
Ambidentate ligand is a ligand which has more than one atom to donate an electron pair but can donate only one pair of electrons. Ambidentate ligands are monodentate ligands with variable donor atoms.
Some ambidentate ligands are $CN^{ - },\quad N{ O }_{ 2 }^{ - },\quad CN{ S }^{ - }$.
Let us consider $CN^{ - }$ ion. Cyanide ion acts as a ligand because of the presence of lone pairs of electrons on both nitrogen and carbon atoms.
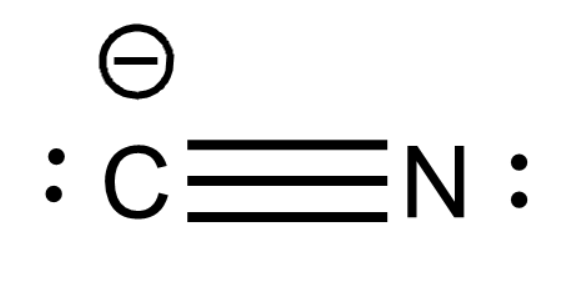
Due to negative charge both carbon and nitrogen atoms have lone pairs of electrons on each of them. And both are capable of donating their lone pair of electrons. But both atoms do not donate their electrons simultaneously.
Note: Though ambidentate ligands have multiple electron donors they cannot form more than one bond because there will be more electron repulsions as 2 electron clouds are very close. And also cyclic structures with small numbered centers are highly unstable.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




