
Sodium oxide ${ Na }_{ 2 }{ O }$ is a crystalline solid, it has:
A. Anti fluorite structure
B. ${ Na }^{ + }$ ions are present at body diagonals
C. ${ Na }^{ + }$ ions are present at tetrahedral voids
D. ${ O }^{ 2- }$ ions are present at octahedral voids
Answer
594k+ views
Hint: A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly, repeating pattern extending in all three spatial dimensions. In crystallography, an anti-fluorite structure is acquired from a salt structure by exchanging anion and cation positions.
Complete step by step answer:
In the antifluorite structure, the anions adopt a face-centered cubic arrangement with cations in the tetrahedral interstices. The cations have a coordination number of 4 and the anions have a coordination number of 8.
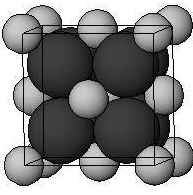
Figure: Cations are shown by black atoms and anions are shown by grey atoms.
In sodium oxide ${ Na }_{ 2 }{ O }$, ${ Na }^{ + }$ ions are present at tetrahedral voids and ${ O }^{ 2- }$ ions occupy half of the cubic holes in the face cubic close lattice. ${ Na }^{ + }$ ions are present at body diagonals.
${ Na }_{ 2 }{ O }$ is an anti fluorite structure.
Hence, the correct answer is option A,B,C.
Additional Information
a.)Voids or interstitial spaces are the gaps present between various spheres of a crystalline solid.
b.)The tetrahedral void is formed when a particle in the second layer is above a void of the first layer.
c.)The octahedral void is formed when a void of the second layer is above the void of the first layer.
d.)There are twice the same number of octahedral gaps as there are tetrahedral openings, in a cross-section.
e.)Body-centered unit cell: When the lattice points are present at the eight corners of the unit cell as well as an additional point at the centre of the cell.
Note:
The possibility to make a mistake is that you may not choose option B. But ${ Na }^{ + }$ ions are present at body diagonals, not on face diagonals.
Complete step by step answer:
In the antifluorite structure, the anions adopt a face-centered cubic arrangement with cations in the tetrahedral interstices. The cations have a coordination number of 4 and the anions have a coordination number of 8.

Figure: Cations are shown by black atoms and anions are shown by grey atoms.
In sodium oxide ${ Na }_{ 2 }{ O }$, ${ Na }^{ + }$ ions are present at tetrahedral voids and ${ O }^{ 2- }$ ions occupy half of the cubic holes in the face cubic close lattice. ${ Na }^{ + }$ ions are present at body diagonals.
${ Na }_{ 2 }{ O }$ is an anti fluorite structure.
Hence, the correct answer is option A,B,C.
Additional Information
a.)Voids or interstitial spaces are the gaps present between various spheres of a crystalline solid.
b.)The tetrahedral void is formed when a particle in the second layer is above a void of the first layer.
c.)The octahedral void is formed when a void of the second layer is above the void of the first layer.
d.)There are twice the same number of octahedral gaps as there are tetrahedral openings, in a cross-section.
e.)Body-centered unit cell: When the lattice points are present at the eight corners of the unit cell as well as an additional point at the centre of the cell.
Note:
The possibility to make a mistake is that you may not choose option B. But ${ Na }^{ + }$ ions are present at body diagonals, not on face diagonals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




