
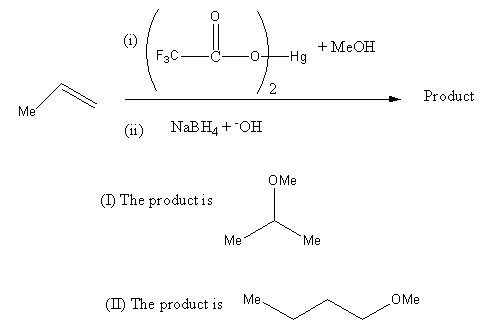
Refer the image and give an answer. Which statement is true?
(i) The product is (\[{\rm I}\])
(ii) The product is (\[{\rm I}\]\[{\rm I}\])
(iii) The reaction is called alkoxymercuration-demercuration(a type of solvomercuration-demercuration).
(iv) It proceeds via markovnikov addition, anti addition of
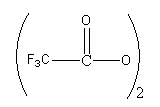 and ROH(a nucleophilic solvent) and no rearrangement.
and ROH(a nucleophilic solvent) and no rearrangement.
(A) (i)
(B) (ii)
(C) (i), (iii), (iv)
(D) (ii), (iii), (iv)


Answer
593.1k+ views
Hint: Markovnikov’s rule states that nucleophilic groups always get attached to more substituted carbon atoms in the addition reaction of alkenes. Mercuric acetate forms three membered rings in the intermediate when it first reacts with the alkene.
Complete answer:
Below is an explanation of how the reaction will occur.
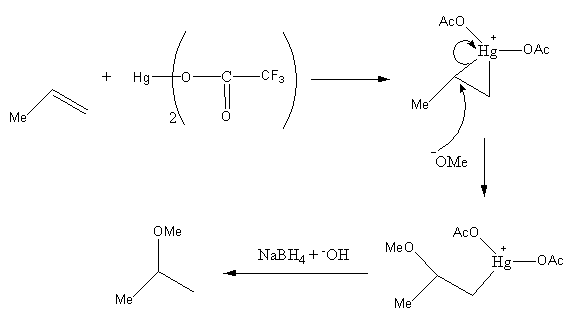
Initially, the double bond will attack the electropositive mercury atom and form a three-membered ring which will get attacked by the nucleophile methanol present in the solution from the back side, which is an anti attack. Then, mercury acetate bonded with carbon atom gets removed when the compound is treated with Sodium borohydride in presence of a base. Hence the product we obtained is 2-methoxy propane.
- In this reaction an alkoxy group and mercury atom gets added to a carbon-carbon double bond, so this reaction is also called alkoxymercuration-demercuration reaction.
- As we can see in the product that nucleophilic group is attached at more stable carbocation, hence it follows Markovnikov’s rule and nucleophile is also ROH.
So, only (ii) is incorrect from above all statements.
So, correct answer is (D) (ii), (iii), (iv)
Additional Information:
-When we need to do addition to alkene and need the Markovnikov product as well as anti addition, then oxymercuration-demercuration reaction is the best way to go.
- As the name suggests, it contains two steps, oxymercuration and demercuration. In the first step, mercury is added in the organic compound and in the second step mercury is removed from the organano-metallic compound.
Note:
- Note that In oxymercuration-demercuration reaction, the addition always follows markovnikov’s rules. So, do not write anti-Markovnikov products as your answer in this reaction.
- Note that base in the second step of the reaction only catalyses the reaction and does not do substitution on the reactant.
Complete answer:
Below is an explanation of how the reaction will occur.

Initially, the double bond will attack the electropositive mercury atom and form a three-membered ring which will get attacked by the nucleophile methanol present in the solution from the back side, which is an anti attack. Then, mercury acetate bonded with carbon atom gets removed when the compound is treated with Sodium borohydride in presence of a base. Hence the product we obtained is 2-methoxy propane.
- In this reaction an alkoxy group and mercury atom gets added to a carbon-carbon double bond, so this reaction is also called alkoxymercuration-demercuration reaction.
- As we can see in the product that nucleophilic group is attached at more stable carbocation, hence it follows Markovnikov’s rule and nucleophile is also ROH.
So, only (ii) is incorrect from above all statements.
So, correct answer is (D) (ii), (iii), (iv)
Additional Information:
-When we need to do addition to alkene and need the Markovnikov product as well as anti addition, then oxymercuration-demercuration reaction is the best way to go.
- As the name suggests, it contains two steps, oxymercuration and demercuration. In the first step, mercury is added in the organic compound and in the second step mercury is removed from the organano-metallic compound.
Note:
- Note that In oxymercuration-demercuration reaction, the addition always follows markovnikov’s rules. So, do not write anti-Markovnikov products as your answer in this reaction.
- Note that base in the second step of the reaction only catalyses the reaction and does not do substitution on the reactant.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




