
Rank in the order of increasing acidity.
I
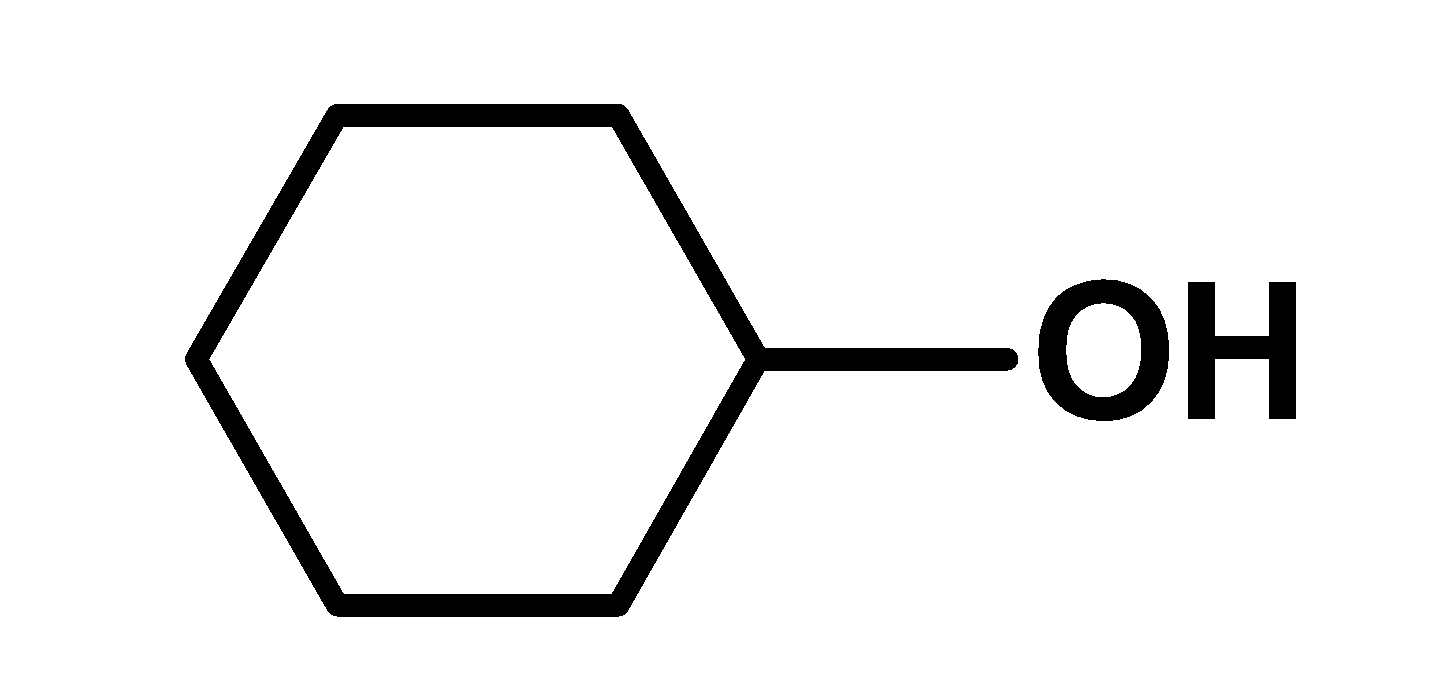
II
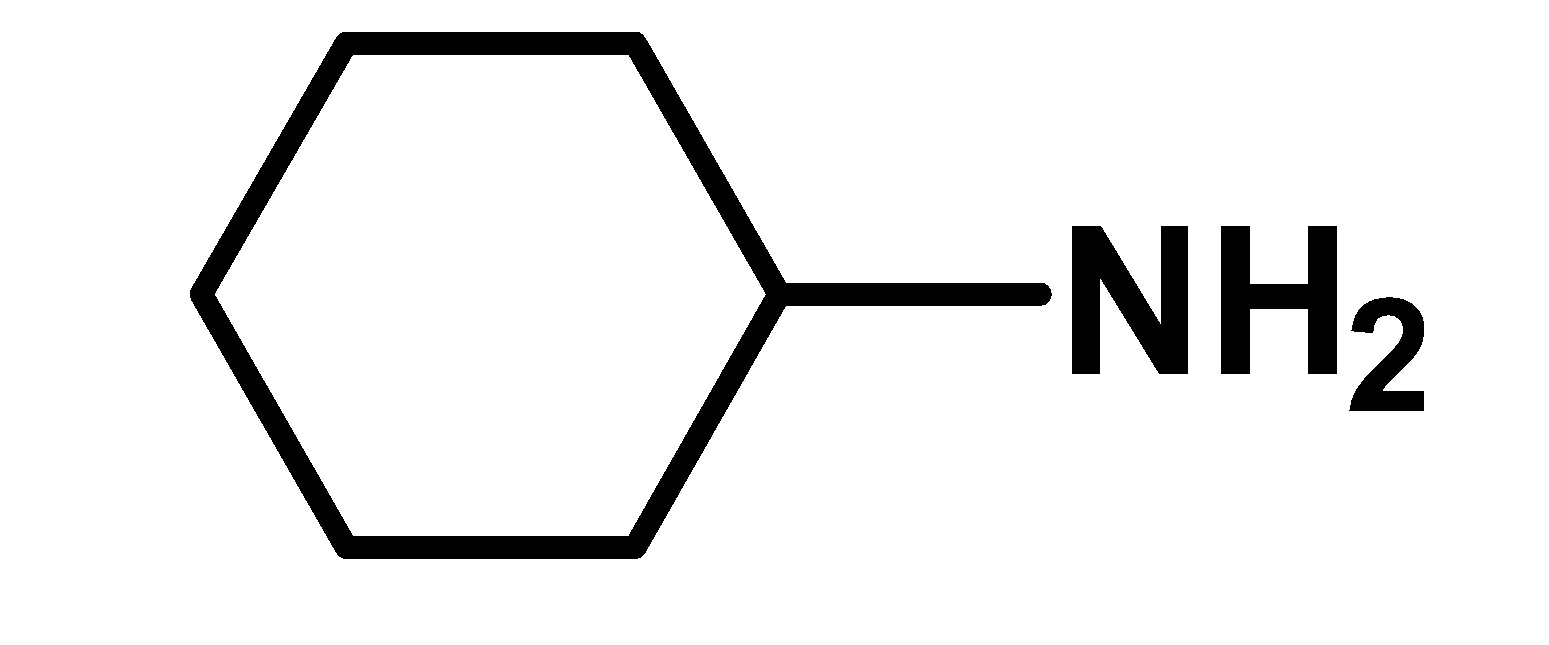
III
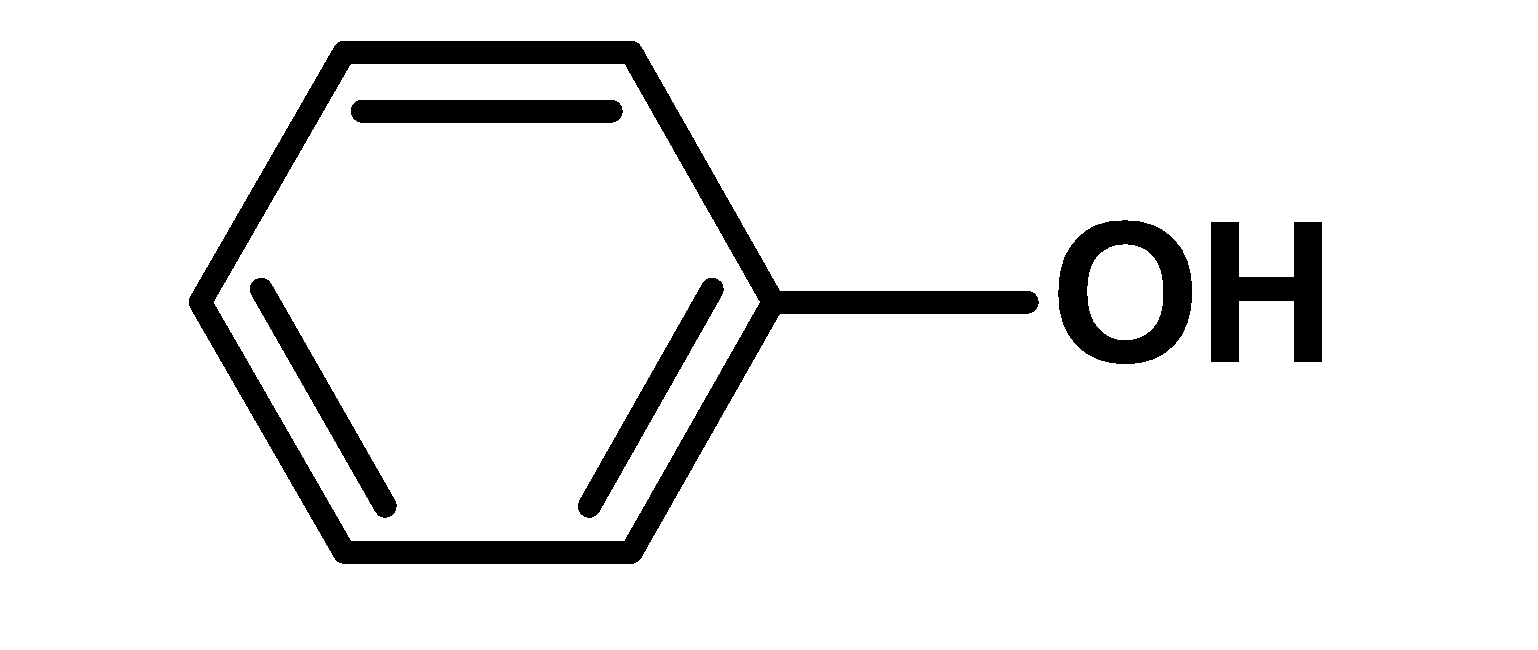
A) $\text{ III }<\text{ I }<\text{ II }$
B) $\text{ I }<\text{ III }<\text{ II }$
C) $\text{ III }<\text{ II }<\text{ I }$
D) $\text{ II }<\text{ I }<\text{ III }$
| I | 
|
| II | 
|
| III | 
|
Answer
573.6k+ views
Hint: acidity is a measure of the extent of loss of proton. The species which can easily donate its proton is more acidic. The acidic characters of organic compounds depend on the polarity of bonds. Similarly, it depends on the stability of anion formed after the loss of a proton. Phenol loses a proton and forms phenoxide ion. Structure of phenoxide ions is stabilized by resonance.
Complete step by step answer:
Phenols are weakly acidic in nature. They turn blue litmus red and react with alkali metal and alkalies to form their salts. The acidic character of phenol is because of the presence of $\text{ O}-\text{H }$ a group. The hydroxyl group in phenol is directly attached to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized carbon atom of benzene. The acidity of the phenol is explained based on resonance stabilized phenoxide ion. Phenoxide is a resonance hybrid of the following structures:
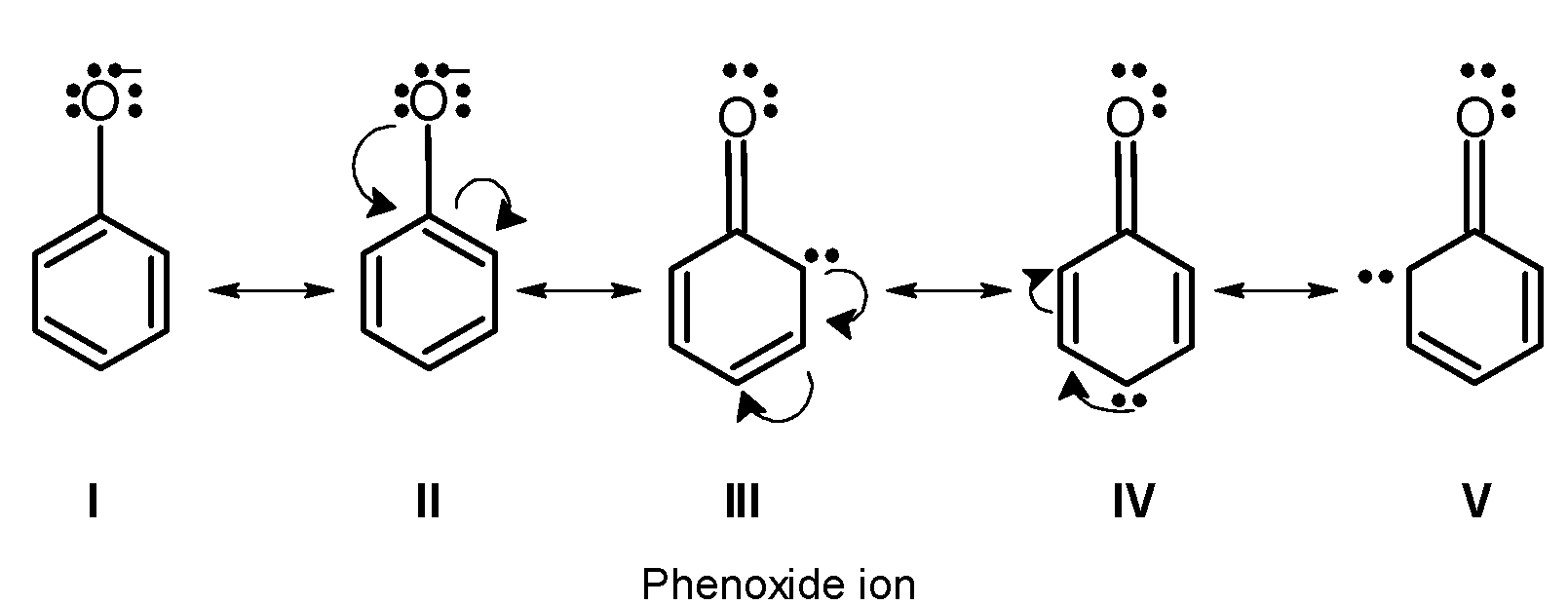
Phenoxide ion is more resonance stabilized than the phenol (undissociated form).there is no structure in the phenoxide ion which requires a large charge separation. Thus resonance stabilized phenoxide ion is more stable. Thus phenol is more acidic. Cyclohexanol is has a hydroxyl group .the hydroxyl group is directly bonded to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$hybridized carbon atom of the cyclohexane ring. This group acts as an electron-withdrawing group. Due to higher electronegativity of oxygen in the$\text{ O}-\text{H }$bond, the electron density moves towards the oxygen. Hydroxyl bond is a polar bond. Thus cyclohexanol loses its proton in the solution. However, cyclohexanol is less acidic than phenol. This is because cyclohexanol is stabilized by the inductive effect only but phenol is stabilized by the resonance effect. Cyclohexylamine is a cyclic amine. The amino $\text{ N}{{\text{H}}_{\text{2}}}\text{ }$ groups are bonded directly to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$hybridized carbon atom. Nitrogen has a lone pair of electrons. We know that Lewis bases are electron donor species. Therefore here Cyclohexylamine is a base and accepts a proton. Cyclohexylamine has a low acidity as compared to phenol and cyclohexanol. Thus increasing order of acidity is, $\text{ II }<\text{ I }<\text{ III }$
Hence, (D) is the correct option.
Note: Note that phenol is more acidic than cyclohexanol and carboxyl amine but it is a weaker acid than carboxylic acid. Therefore like the carboxylic acid, phenol does not react with sodium carbonate and sodium bicarbonate. But they are more acidic than the phenol.
Complete step by step answer:
Phenols are weakly acidic in nature. They turn blue litmus red and react with alkali metal and alkalies to form their salts. The acidic character of phenol is because of the presence of $\text{ O}-\text{H }$ a group. The hydroxyl group in phenol is directly attached to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized carbon atom of benzene. The acidity of the phenol is explained based on resonance stabilized phenoxide ion. Phenoxide is a resonance hybrid of the following structures:

Phenoxide ion is more resonance stabilized than the phenol (undissociated form).there is no structure in the phenoxide ion which requires a large charge separation. Thus resonance stabilized phenoxide ion is more stable. Thus phenol is more acidic. Cyclohexanol is has a hydroxyl group .the hydroxyl group is directly bonded to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$hybridized carbon atom of the cyclohexane ring. This group acts as an electron-withdrawing group. Due to higher electronegativity of oxygen in the$\text{ O}-\text{H }$bond, the electron density moves towards the oxygen. Hydroxyl bond is a polar bond. Thus cyclohexanol loses its proton in the solution. However, cyclohexanol is less acidic than phenol. This is because cyclohexanol is stabilized by the inductive effect only but phenol is stabilized by the resonance effect. Cyclohexylamine is a cyclic amine. The amino $\text{ N}{{\text{H}}_{\text{2}}}\text{ }$ groups are bonded directly to the $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$hybridized carbon atom. Nitrogen has a lone pair of electrons. We know that Lewis bases are electron donor species. Therefore here Cyclohexylamine is a base and accepts a proton. Cyclohexylamine has a low acidity as compared to phenol and cyclohexanol. Thus increasing order of acidity is, $\text{ II }<\text{ I }<\text{ III }$
Hence, (D) is the correct option.
Note: Note that phenol is more acidic than cyclohexanol and carboxyl amine but it is a weaker acid than carboxylic acid. Therefore like the carboxylic acid, phenol does not react with sodium carbonate and sodium bicarbonate. But they are more acidic than the phenol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




