
Question:
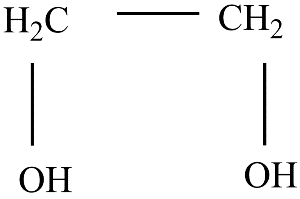
on heating with periodic acid gives:
(A)
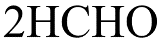
(B)

(C)
(D)



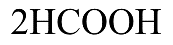

Answer
517.2k+ views
Hint : The compound with molecular formula $ {\left( {C{H_2}OH} \right)_2} $ is called Ethylene glycol. Its IUPAC name is $ ethane - 1,2 - diol. $ It has two hydroxyl groups. Periodic acid has the molecular formula $ HI{O_4}. $ It is a strong oxidizing agent and is usually used to oxidize glycols. An alcohol on oxidation usually leads to the formation of an aldehyde.
Complete Step By Step Answer:
The reaction of ethylene glycol with periodic acid is depicted as follows:
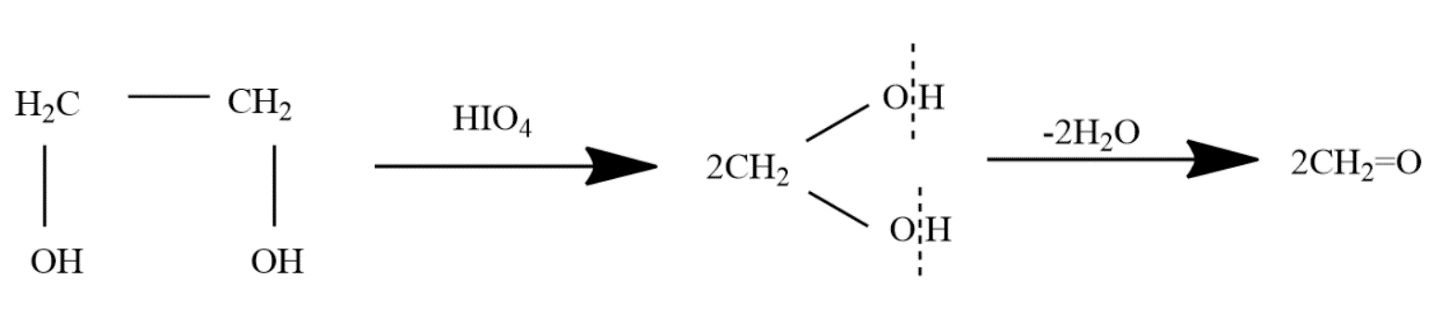
Here, ethylene glycol gets oxidized by periodic acid. The steps are as follows:
- Ethylene glycol reacts with periodic acid and the carbon-carbon bond is broken
- Two molecules of an unstable intermediate are formed in which two hydroxyl groups are attached to a single carbon atom
- The unstable intermediate quickly loses two molecules of water to give formaldehyde as the product.
The reaction is called an oxidation reaction as ethylene glycol, a diol, is converted to aldehyde.
Therefore, on heating $ {\left( {C{H_2}OH} \right)_2} $ with periodic acid gives (a) $2HCHO$.
Note :
Formaldehyde can be written as $ C{H_2} = O $ or $ HCHO, $ both are the same. The oxygen atom is doubly bonded to the carbon which is saturated with two hydrogen atoms. Periodic acid is the highest oxoacid of Iodine. An oxoacid is an acid which has at least one oxygen atom in it. In periodic acid, Iodine exists in the oxidation state $ + 7. $ It is an inorganic acid and can exist in two forms: one being orthoperiodic form, and the other being meta periodic form. Another point to note is that alcohols in the presence of strong oxidizing agents like $ KMn{O_4} $ yield carboxylic acids directly.
Complete Step By Step Answer:
The reaction of ethylene glycol with periodic acid is depicted as follows:

Here, ethylene glycol gets oxidized by periodic acid. The steps are as follows:
- Ethylene glycol reacts with periodic acid and the carbon-carbon bond is broken
- Two molecules of an unstable intermediate are formed in which two hydroxyl groups are attached to a single carbon atom
- The unstable intermediate quickly loses two molecules of water to give formaldehyde as the product.
The reaction is called an oxidation reaction as ethylene glycol, a diol, is converted to aldehyde.
Therefore, on heating $ {\left( {C{H_2}OH} \right)_2} $ with periodic acid gives (a) $2HCHO$.
Note :
Formaldehyde can be written as $ C{H_2} = O $ or $ HCHO, $ both are the same. The oxygen atom is doubly bonded to the carbon which is saturated with two hydrogen atoms. Periodic acid is the highest oxoacid of Iodine. An oxoacid is an acid which has at least one oxygen atom in it. In periodic acid, Iodine exists in the oxidation state $ + 7. $ It is an inorganic acid and can exist in two forms: one being orthoperiodic form, and the other being meta periodic form. Another point to note is that alcohols in the presence of strong oxidizing agents like $ KMn{O_4} $ yield carboxylic acids directly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




