
Propyne and propene can be distinguished by:
A. Conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$
B. $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$
C. Dilute $\text{KMn}{{\text{O}}_{4}}$
D. $\text{AgN}{{\text{O}}_{3}}$ in ammonia
Answer
232.8k+ views
Hint: The chemical reagents give differences in chemical compounds. Concentrated sulphuric acid and bromination show additional reactions. Dilute potassium permanganate gives carboxylic acids on reaction with one and alcohols with another. Ammoniacal silver nitrate removes acidic hydrogen to give precipitates.
Complete step by step solution:
Let us discuss the products formed when propyne and propene react with the reagents given in the options:
(A) Conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$: Propyne reacts with concentrated sulphuric acid in presence of mercuric sulphate to form acetone in equilibrium with its tautomeric structure. The reaction is
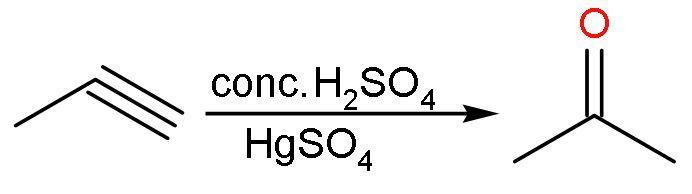
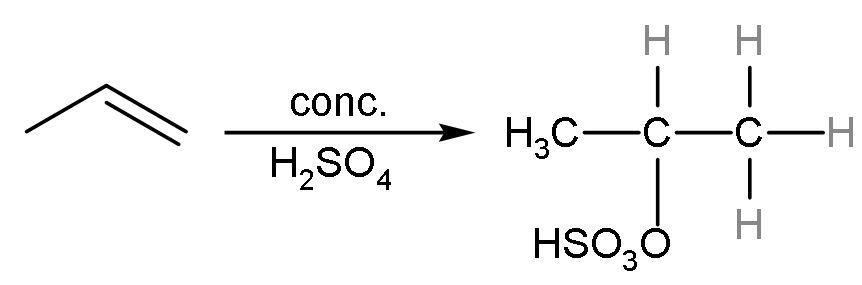
Propene reacts with concentrated sulphuric acid to give an additional reaction. In this reaction, ${{\text{H}}^{+}}$ of sulphuric acid adds to more hydrogenated double-bonded carbon and $^{-}\text{OS}{{\text{O}}_{3}}\text{H}$ to less hydrogenated carbon atom. The reaction is
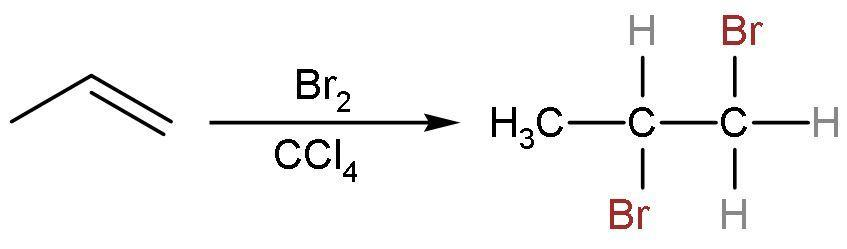
(B) $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$: Propene is an unsaturated compound. So, it gives an additional reaction with bromine in a nonpolar solvent of carbon tetrachloride. The double bond is replaced by the single bond between the carbon atoms and additions of bromine atoms on both the double-bonded carbon atoms take place. The reaction is
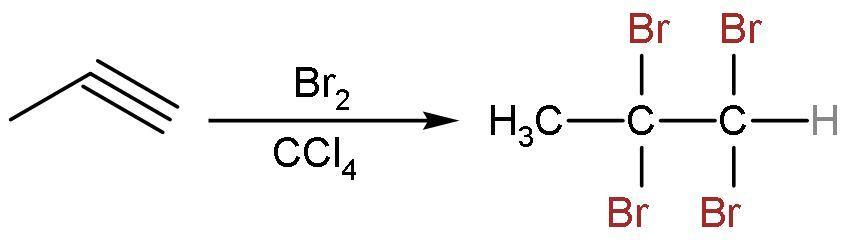
Similarly, propyne reacts with $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$ to give addition reaction only. Here, the triple bond is replaced by the single bond. The 2 moles of $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$ will be needed here. The reaction is
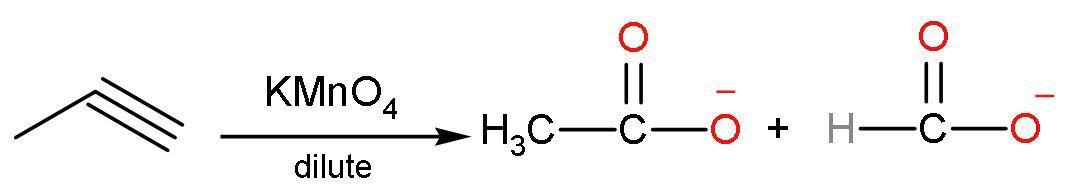
(C) Dilute $\text{KMn}{{\text{O}}_{4}}$: In this reaction, propyne reacts with potassium permanganate to give carboxylic acids of lesser carbon atoms. It means that the triple bond is broken from the presence of a triple bond. The reaction is
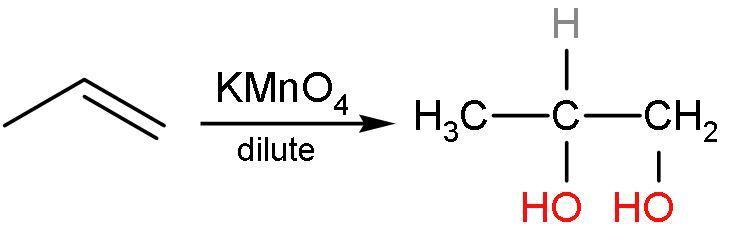
Propene reacts with potassium permanganate to give an additional reaction. In this, the hydroxyl groups are added to both the double-bonded carbon atom. This is vicinal diols or 1,2-diols. The double bond is replaced by the alcohol groups. The reaction is
(D) $\text{AgN}{{\text{O}}_{3}}$ in ammonia: Propene does not react with ammoniacal silver nitrate.
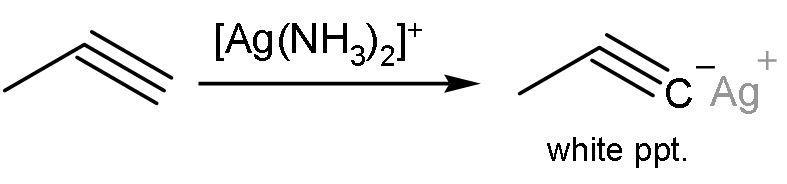
Propyne, on the other hand, gives this reaction. Propyne has a triple bond between ${{\text{C}}_{1}}-{{\text{C}}_{2}}$. This means that the alkyne is terminal. Terminal alkynes have acidic hydrogens. These hydrogens are removed and insoluble precipitates are formed. The reaction is
Propyne and propene can be distinguished by $\text{AgN}{{\text{O}}_{3}}$ in ammonia, which is option ’d’.
So, the connected answer is (D).
Note: Aldehydes are oxidised by diamine silver ions $\left[ \text{Ag}\left( \text{N}{{\text{H}}_{3}} \right)_{2}^{+} \right]$ or ammoniacal silver nitrate solution to carboxylic acids. This reagent is also known as Tollen’s reagent. The reaction forms out of a silver mirror as a product to confirm the presence of aldehydes. The reaction is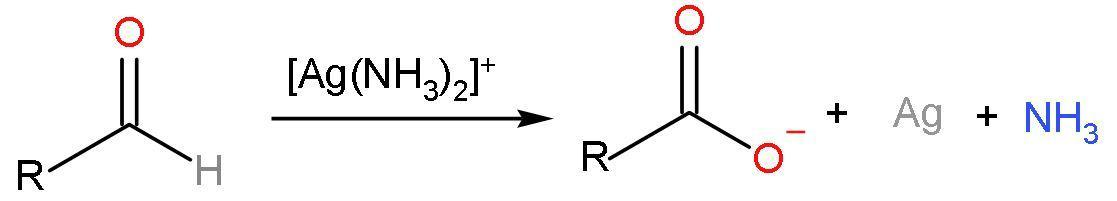
Complete step by step solution:
Let us discuss the products formed when propyne and propene react with the reagents given in the options:
(A) Conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$: Propyne reacts with concentrated sulphuric acid in presence of mercuric sulphate to form acetone in equilibrium with its tautomeric structure. The reaction is
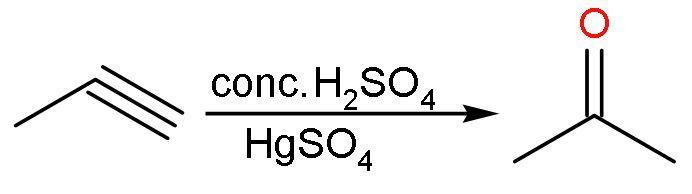
Propene reacts with concentrated sulphuric acid to give an additional reaction. In this reaction, ${{\text{H}}^{+}}$ of sulphuric acid adds to more hydrogenated double-bonded carbon and $^{-}\text{OS}{{\text{O}}_{3}}\text{H}$ to less hydrogenated carbon atom. The reaction is

(B) $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$: Propene is an unsaturated compound. So, it gives an additional reaction with bromine in a nonpolar solvent of carbon tetrachloride. The double bond is replaced by the single bond between the carbon atoms and additions of bromine atoms on both the double-bonded carbon atoms take place. The reaction is

Similarly, propyne reacts with $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$ to give addition reaction only. Here, the triple bond is replaced by the single bond. The 2 moles of $\text{B}{{\text{r}}_{2}}$ in $\text{CC}{{\text{l}}_{4}}$ will be needed here. The reaction is

(C) Dilute $\text{KMn}{{\text{O}}_{4}}$: In this reaction, propyne reacts with potassium permanganate to give carboxylic acids of lesser carbon atoms. It means that the triple bond is broken from the presence of a triple bond. The reaction is

Propene reacts with potassium permanganate to give an additional reaction. In this, the hydroxyl groups are added to both the double-bonded carbon atom. This is vicinal diols or 1,2-diols. The double bond is replaced by the alcohol groups. The reaction is

(D) $\text{AgN}{{\text{O}}_{3}}$ in ammonia: Propene does not react with ammoniacal silver nitrate.
Propyne, on the other hand, gives this reaction. Propyne has a triple bond between ${{\text{C}}_{1}}-{{\text{C}}_{2}}$. This means that the alkyne is terminal. Terminal alkynes have acidic hydrogens. These hydrogens are removed and insoluble precipitates are formed. The reaction is

Propyne and propene can be distinguished by $\text{AgN}{{\text{O}}_{3}}$ in ammonia, which is option ’d’.
So, the connected answer is (D).
Note: Aldehydes are oxidised by diamine silver ions $\left[ \text{Ag}\left( \text{N}{{\text{H}}_{3}} \right)_{2}^{+} \right]$ or ammoniacal silver nitrate solution to carboxylic acids. This reagent is also known as Tollen’s reagent. The reaction forms out of a silver mirror as a product to confirm the presence of aldehydes. The reaction is

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




