
Propane $\to $ 2-Iodopropane
Best reagent for the above conversion is:
(a)- ${{I}_{2}}/hv$
(b)- NaI / acetone
(c)- ${{F}_{2}}/hv$, NaI / acetone
(d)- $B{{r}_{2}}/hv$, NaI / acetone
Answer
565.5k+ views
Hint: When alkane is treated with halogen, sunlight is used as a catalyst for the addition of halogen. But the fluorine and iodine cannot be directly attached to the alkane by using sunlight.
Complete answer:
Propane is an organic compound in which the number of carbon atoms is three and there are only single bonds in this compound. The formula is:
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$
So this compound can undergo substitution reaction by the replacement of one of the hydrogens from the carbon atom and then replacing it with another atom or molecule. So in propane, there are two places in which the incoming molecule can attack, i.e., either on the terminal carbon atoms or at the middle carbon atom. If the incoming molecule is nucleophile then it will probably attach the middle carbon atom, because the intermediate will be secondary carbocation.
So, when propane is treated with any halogen in the presence of sunlight, then one hydrogen atom at the second place will be replaced with the halogen atom, leading to the formation of 2-halopropane.
But this reaction is only given by chlorine and bromine. So if iodine has to be substituted with the hydrogen atom, first, we have to convert the alkane into 2-bromopropane and then substituting it with the iodine atom. So the reaction takes place as follows:
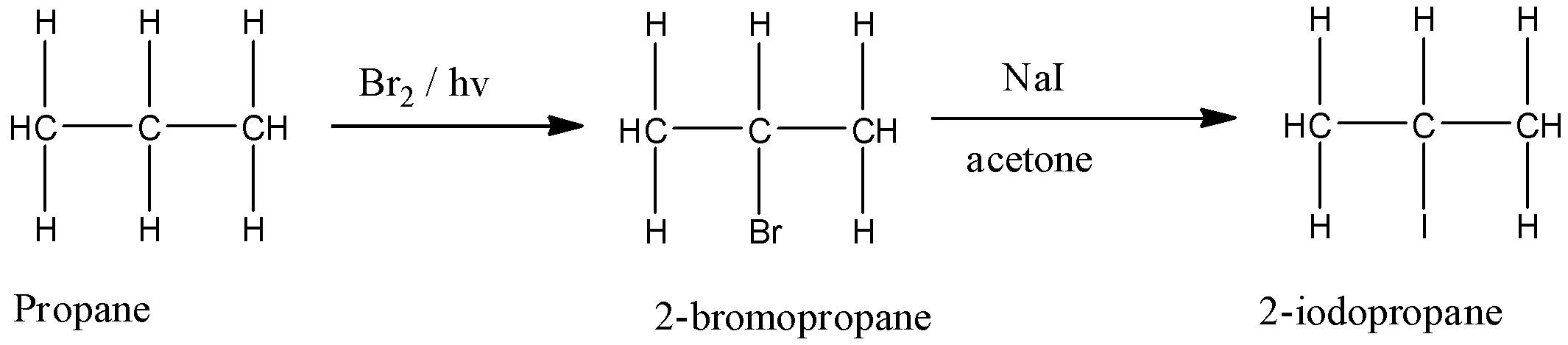
So the correct reagents used are $B{{r}_{2}}/hv$, NaI / acetone.
Therefore, the correct answer is an option (d)- $B{{r}_{2}}/hv$, NaI / acetone.
Note:
Fluorine and iodine cannot be used in the reaction because in the presence of sunlight, the reaction with fluorine is very vigorous and explosive, and the reaction of iodine is reversible and doesn’t form the iodoalkane.
Complete answer:
Propane is an organic compound in which the number of carbon atoms is three and there are only single bonds in this compound. The formula is:
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$
So this compound can undergo substitution reaction by the replacement of one of the hydrogens from the carbon atom and then replacing it with another atom or molecule. So in propane, there are two places in which the incoming molecule can attack, i.e., either on the terminal carbon atoms or at the middle carbon atom. If the incoming molecule is nucleophile then it will probably attach the middle carbon atom, because the intermediate will be secondary carbocation.
So, when propane is treated with any halogen in the presence of sunlight, then one hydrogen atom at the second place will be replaced with the halogen atom, leading to the formation of 2-halopropane.
But this reaction is only given by chlorine and bromine. So if iodine has to be substituted with the hydrogen atom, first, we have to convert the alkane into 2-bromopropane and then substituting it with the iodine atom. So the reaction takes place as follows:

So the correct reagents used are $B{{r}_{2}}/hv$, NaI / acetone.
Therefore, the correct answer is an option (d)- $B{{r}_{2}}/hv$, NaI / acetone.
Note:
Fluorine and iodine cannot be used in the reaction because in the presence of sunlight, the reaction with fluorine is very vigorous and explosive, and the reaction of iodine is reversible and doesn’t form the iodoalkane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




