
Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give:
(A) Phenolphthalein
(B) alizarin
(C) coumarin
(D) fluorescein
Answer
581.4k+ views
Hint: Phthalic acid is a dicarboxylic acid. It reacts with a dehydrating agent i.e. sulphuric acid. Phthalic acid loses water on heating and acts as a dehydrating agent. The reaction of phthalic anhydride with resorcinol results in the formation of organic dye. This dye has a wide application as a fluorescent material in the laser application.
Complete Solution :
We have been provided that phthalic acid with resorcinol in the presence of concentrated sulphuric acid,
Phthalic acid is an aromatic dicarboxylic acid, with formula ${{C}_{6}}{{H}_{4}}{{(C{{O}_{2}}H)}_{2}}$ .It is an isomer of isophthalic acid and terephthalic acid. Resorcinol is an organic compound with the formula ${{C}_{6}}{{H}_{4}}{{(OH)}_{2}}$. It is one of three isomeric benzenediols.
- When these two compounds react together in presence of concentrated sulphuric acid,
Sulphuric acid acts as a dehydrating agent because a substance that absorbs moisture from its surroundings is called a dehydrating agent. Sulphuric acid readily protonated ${{H}_{2}}O$ leading to the formation of hydronium ions.
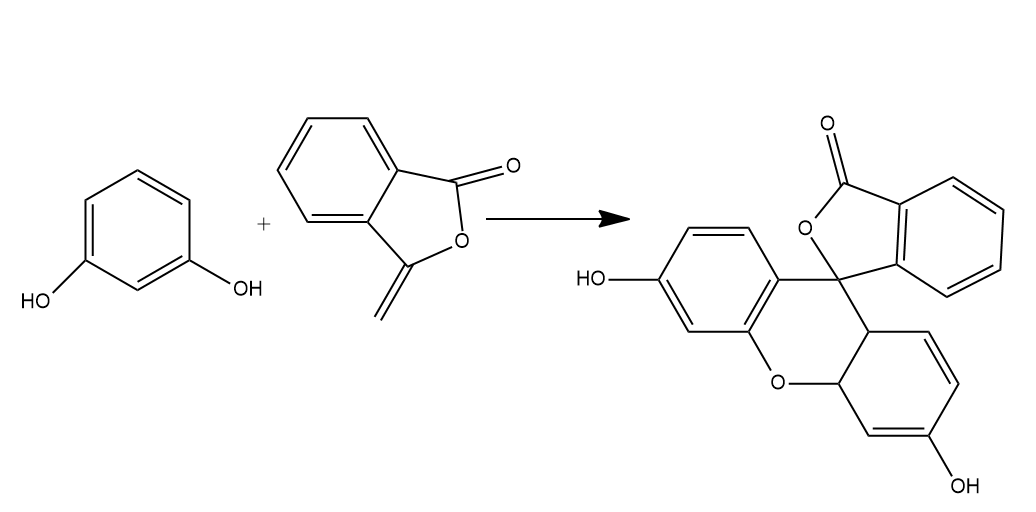
- Phthalic acid reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein. Phthalic acid gets dehydrated on treatment with concentrated ${{H}_{2}}S{{O}_{4}}$ to form phthalic anhydride. Phthalic anhydride further reacts with resorcinol to form fluorescein.
- So, we can say that Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein.
So, the correct answer is “Option D”.
Note: Note that Adolf Von Baeyer first synthesized fluorescein in the laboratory. He prepared it from the phallic anhydride and resorcinol in presence of zinc chloride through the Friedel-Crafts reaction. There is one more method for synthesis using methane sulphonic acid . This gives a high yield.
Sulphuric acid is not used as a drying agent ${{H}_{2}}S$ because it reacts with it to form sulphur.
$\text{ }{{H}_{2}}S{{O}_{4}}+{{H}_{2}}S\to 2{{H}_{2}}O+S{{O}_{2}}+S\text{ }$
Complete Solution :
We have been provided that phthalic acid with resorcinol in the presence of concentrated sulphuric acid,
Phthalic acid is an aromatic dicarboxylic acid, with formula ${{C}_{6}}{{H}_{4}}{{(C{{O}_{2}}H)}_{2}}$ .It is an isomer of isophthalic acid and terephthalic acid. Resorcinol is an organic compound with the formula ${{C}_{6}}{{H}_{4}}{{(OH)}_{2}}$. It is one of three isomeric benzenediols.
- When these two compounds react together in presence of concentrated sulphuric acid,
Sulphuric acid acts as a dehydrating agent because a substance that absorbs moisture from its surroundings is called a dehydrating agent. Sulphuric acid readily protonated ${{H}_{2}}O$ leading to the formation of hydronium ions.

- Phthalic acid reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein. Phthalic acid gets dehydrated on treatment with concentrated ${{H}_{2}}S{{O}_{4}}$ to form phthalic anhydride. Phthalic anhydride further reacts with resorcinol to form fluorescein.
- So, we can say that Phthalic anhydride reacts with resorcinol in the presence of concentrated ${{H}_{2}}S{{O}_{4}}$ to give fluorescein.
So, the correct answer is “Option D”.
Note: Note that Adolf Von Baeyer first synthesized fluorescein in the laboratory. He prepared it from the phallic anhydride and resorcinol in presence of zinc chloride through the Friedel-Crafts reaction. There is one more method for synthesis using methane sulphonic acid . This gives a high yield.
Sulphuric acid is not used as a drying agent ${{H}_{2}}S$ because it reacts with it to form sulphur.
$\text{ }{{H}_{2}}S{{O}_{4}}+{{H}_{2}}S\to 2{{H}_{2}}O+S{{O}_{2}}+S\text{ }$
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




