
What is the oxidation state of Cobalt in the following molecule?
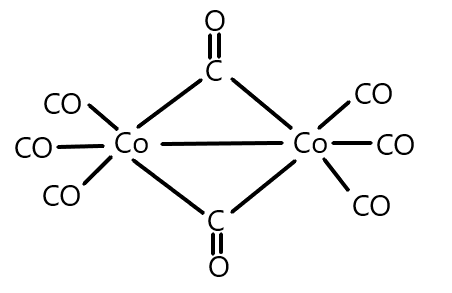
A) $3$
B) $1$
C) $2$
D) $0$

Answer
565.8k+ views
Hint: As we know that oxidation state or oxidation number of the central metal atom/ion in a complex is the charge present on that atom/ ion if all the ligands are removed along with the electron pairs that are shared by the central atom/ion.
Complete step by step answer:
As we know that a ligand is that donor atom or molecule or anion which donates a pair of electrons to the metal atom/ion and hence they are also called as lewis bases.
We also know that complexes can be homoleptic and heteroleptic where homoleptic complexes are those which possess only one type of ligand and heteroleptic complexes are those that possess more than one type of ligands.
We are also aware of the oxidation state of the metal atom of the central atom which is given by the charge present on it.
Now, in the given molecule, we can see that the ligand present is the Carbonyl group which we know that is a neutral ligand and thus possesses zero charge. A total of eight carbonyl groups are present as ligands which will contribute a zero charge on the central atom that is cobalt because it is a homoleptic complex and no other ligand is present. And we can write the complex as: $[Co{(CO)_8}]$
Thus, from the above calculation the correct answer is (D).
Note: Always remember that carbonyl and cobalt seem similar but carbonyl contains $CO$ both in capital and it is a neutral ligand whereas $Co$ is a metal atom Cobalt. A neutral ligand is the one which does not contain any charge and electron pairs to donate. Along with $CO$, $N{H_3}$ and ${H_2}O$ are also neutral ligands. Halogens are considered anionic ligands and positively charged ligands are cationic ligands.
Complete step by step answer:
As we know that a ligand is that donor atom or molecule or anion which donates a pair of electrons to the metal atom/ion and hence they are also called as lewis bases.
We also know that complexes can be homoleptic and heteroleptic where homoleptic complexes are those which possess only one type of ligand and heteroleptic complexes are those that possess more than one type of ligands.
We are also aware of the oxidation state of the metal atom of the central atom which is given by the charge present on it.
Now, in the given molecule, we can see that the ligand present is the Carbonyl group which we know that is a neutral ligand and thus possesses zero charge. A total of eight carbonyl groups are present as ligands which will contribute a zero charge on the central atom that is cobalt because it is a homoleptic complex and no other ligand is present. And we can write the complex as: $[Co{(CO)_8}]$
Thus, from the above calculation the correct answer is (D).
Note: Always remember that carbonyl and cobalt seem similar but carbonyl contains $CO$ both in capital and it is a neutral ligand whereas $Co$ is a metal atom Cobalt. A neutral ligand is the one which does not contain any charge and electron pairs to donate. Along with $CO$, $N{H_3}$ and ${H_2}O$ are also neutral ligands. Halogens are considered anionic ligands and positively charged ligands are cationic ligands.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




