
Osmotic pressure observed when benzoic acid is dissolved in benzene, is less than that expected from theoretical considerations. This is because:
A.) Benzoic acid is organic solute.
B.) Benzoic acid has higher molar mass than benzene.
C.) Benzoic acid gets associated in benzene.
D.) Benzoic acid gets dissociated in benzene.
Answer
594k+ views
Hint: It will be defined by considering the properties of the benzoic acid in benzene mainly related to the structure of molecules. We should also keep in mind that osmotic pressure is a colligative property which depends on the number of solutes.
Complete answer:
First, let us know about the osmotic pressure. It is defined in terms of solution, considered as a colligative property.
We can define the osmotic pressure as the pressure required for the movement of solvent particles via a semipermeable membrane, and this process is known as osmosis.
Thus, in case of molecules, osmotic pressure is described in terms of their existence, the number of molecules. Benzoic acid in benzene solution is subjected to extensive dimerization. It is due to hydrogen bonding between the lone pair of oxygen atom on benzoic acid and hydrogen atom on another benzoic acid. This bonding is also favoured thermodynamically and makes the bonding strong and stable as compared to the single benzoic acid. It leads to form extensive connectivity in the molecule and undergoes association. The diagrammatic representation is shown below-
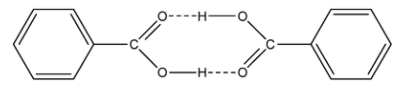
Due to association of molecules of benzoic acid, the effective number of particles will be halved and mass of one particle will be doubled from the theoretical value.
> If we talk about the first option, i.e. benzoic acid is organic solute. Benzoic acid is an organic compound but mostly used as a solvent.
> The second option is regarding the molar mass, then we can say that molar mass doesn’t show any effect on osmotic pressure.
> Now talking about the third option, it means that benzoic acid is associated with the benzene, because it exists as a dimer. Thus, it will lead to the decrease in number of molecules, and further osmotic pressure also decreases.
> The fourth option is about the dissociation which cannot be considered.
In the last, we can conclude that the variation is because the benzoic acid gets associated with benzene.
Hence, the correct option is (C).
Note: We have to relate the point in terms of molar concentration. So, as mentioned we considered the number of molecules directly relating to the osmotic pressure in case of benzene, and benzoic acid. Also, the stoichiometric coefficient (n) is 2 as benzoic acid forms 2 intermolecular hydrogen bonds within themselves.
Complete answer:
First, let us know about the osmotic pressure. It is defined in terms of solution, considered as a colligative property.
We can define the osmotic pressure as the pressure required for the movement of solvent particles via a semipermeable membrane, and this process is known as osmosis.
Thus, in case of molecules, osmotic pressure is described in terms of their existence, the number of molecules. Benzoic acid in benzene solution is subjected to extensive dimerization. It is due to hydrogen bonding between the lone pair of oxygen atom on benzoic acid and hydrogen atom on another benzoic acid. This bonding is also favoured thermodynamically and makes the bonding strong and stable as compared to the single benzoic acid. It leads to form extensive connectivity in the molecule and undergoes association. The diagrammatic representation is shown below-

Due to association of molecules of benzoic acid, the effective number of particles will be halved and mass of one particle will be doubled from the theoretical value.
> If we talk about the first option, i.e. benzoic acid is organic solute. Benzoic acid is an organic compound but mostly used as a solvent.
> The second option is regarding the molar mass, then we can say that molar mass doesn’t show any effect on osmotic pressure.
> Now talking about the third option, it means that benzoic acid is associated with the benzene, because it exists as a dimer. Thus, it will lead to the decrease in number of molecules, and further osmotic pressure also decreases.
> The fourth option is about the dissociation which cannot be considered.
In the last, we can conclude that the variation is because the benzoic acid gets associated with benzene.
Hence, the correct option is (C).
Note: We have to relate the point in terms of molar concentration. So, as mentioned we considered the number of molecules directly relating to the osmotic pressure in case of benzene, and benzoic acid. Also, the stoichiometric coefficient (n) is 2 as benzoic acid forms 2 intermolecular hydrogen bonds within themselves.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




