
On commercial scale, phenol is obtained from chlorobenzene. The chlorobenzene needed for the purpose is prepared by:
A.

B.
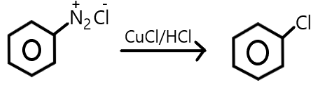
C.
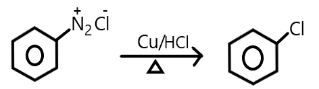
D.
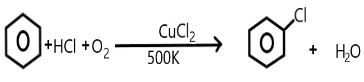




Answer
553.2k+ views
Hint: As we know that arenes undergo electrophilic aromatic substitution reaction with chlorine, bromine and iodine in the presence of lewis acid to given haloarenes which on oxidation can give phenol as the major product but it can also be obtained by the Raschig’s process mixture of benzene vapours.
Complete answer:
We are very well aware of the process of Raschig and Hooker where the production of phenol takes place. The main steps involved in this process includes the formation of chlorobenzene from the benzene along with hydrochloric acid and oxygen present in the air.
So, the subsequent hydrolysis of this chlorobenzene so formed results in the production of phenol in the presence of copper chloride at a temperature of about $500K$.
Copper chloride acts as a catalyst in this process at the first step that is while formation of chlorobenzene takes place from the benzene.
In the second step, the chlorobenzene is subjected to steam at a temperature of about $723K$ over a silicon catalyst which basically hydrolyses it to become phenol and hydrochloric acid.
This hydrochloric acid can be recycled back to use in the reactant or the first step as a reactant.
We can show this whole process of phenol production as:
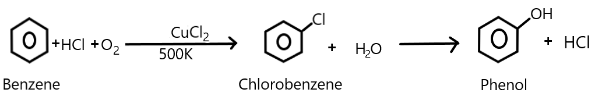
Therefore the correct answer is (D).
Note: Chlorobenzene can be formed diazonium salt in the presence of copper chloride and copper/ hydrochloride but they are not commercially used rather they are used in laboratories only for small purposes. Raschig’s process is commercially efficient to obtain the phenol from the chlorobenzene.
Complete answer:
We are very well aware of the process of Raschig and Hooker where the production of phenol takes place. The main steps involved in this process includes the formation of chlorobenzene from the benzene along with hydrochloric acid and oxygen present in the air.
So, the subsequent hydrolysis of this chlorobenzene so formed results in the production of phenol in the presence of copper chloride at a temperature of about $500K$.
Copper chloride acts as a catalyst in this process at the first step that is while formation of chlorobenzene takes place from the benzene.
In the second step, the chlorobenzene is subjected to steam at a temperature of about $723K$ over a silicon catalyst which basically hydrolyses it to become phenol and hydrochloric acid.
This hydrochloric acid can be recycled back to use in the reactant or the first step as a reactant.
We can show this whole process of phenol production as:

Therefore the correct answer is (D).
Note: Chlorobenzene can be formed diazonium salt in the presence of copper chloride and copper/ hydrochloride but they are not commercially used rather they are used in laboratories only for small purposes. Raschig’s process is commercially efficient to obtain the phenol from the chlorobenzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




