
How is Nylon 6,6 obtained. Give reaction
Answer
600.3k+ views
Hint:Nylon 6,6 is a type of polyamide or nylon. It is made up of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid.
Complete step by step answer:
Nylon,6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid.
Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/ carbonate mixture and thus molten nylon,6,6 is formed.
To prepare Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is a shown:
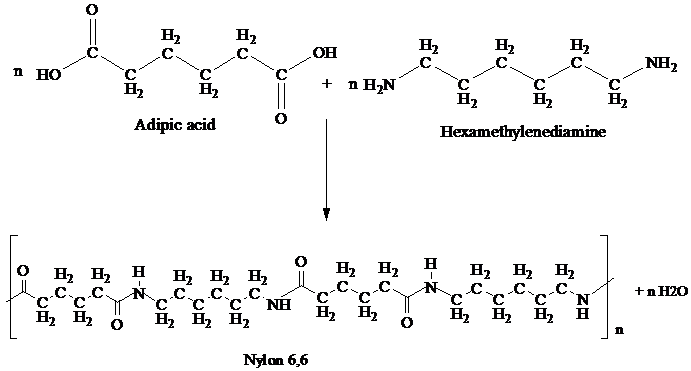
The resulting polymer is extruded into a wide range of fiber types. The fibers are drawn, or sketched in a process that increases their length and reorients the materials molecules parallel to one another to produce a strong, elastic filament.
Note:
Nylon 6,6 has long molecular chains resulting in more hydrogen bonds, creating chemical springs and making it very resilient. It is an amorphous solid so it has a large elastic property and is slightly soluble in boiling water and is very stable in nature. It is very difficult to dye, but one it is dyed it has a high colorfastness and is less susceptible to fading.
Complete step by step answer:
Nylon,6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid.
Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/ carbonate mixture and thus molten nylon,6,6 is formed.
To prepare Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is a shown:
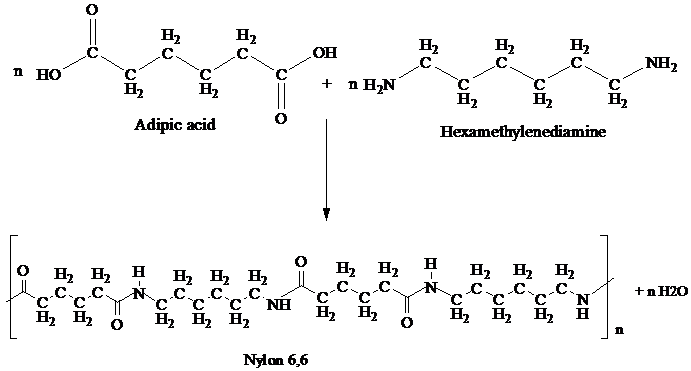
The resulting polymer is extruded into a wide range of fiber types. The fibers are drawn, or sketched in a process that increases their length and reorients the materials molecules parallel to one another to produce a strong, elastic filament.
Note:
Nylon 6,6 has long molecular chains resulting in more hydrogen bonds, creating chemical springs and making it very resilient. It is an amorphous solid so it has a large elastic property and is slightly soluble in boiling water and is very stable in nature. It is very difficult to dye, but one it is dyed it has a high colorfastness and is less susceptible to fading.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




