
Number of $Cr - O$ bonds in dichromate ion $\left( {C{r_2}{O_7}^{2 - }} \right)$ is.
A) $6$
B) $7$
C) $8$
D) $4$
Answer
578.4k+ views
Hint: We know that there are two chromium atoms and seven oxygen atoms are present in dichromate anion and the each chromium atom is linked to three oxygen atoms and there is a bridge between the chromium atom and the one oxygen atom.
Complete step by step answer:
Now we see the structure of dichromate ion,
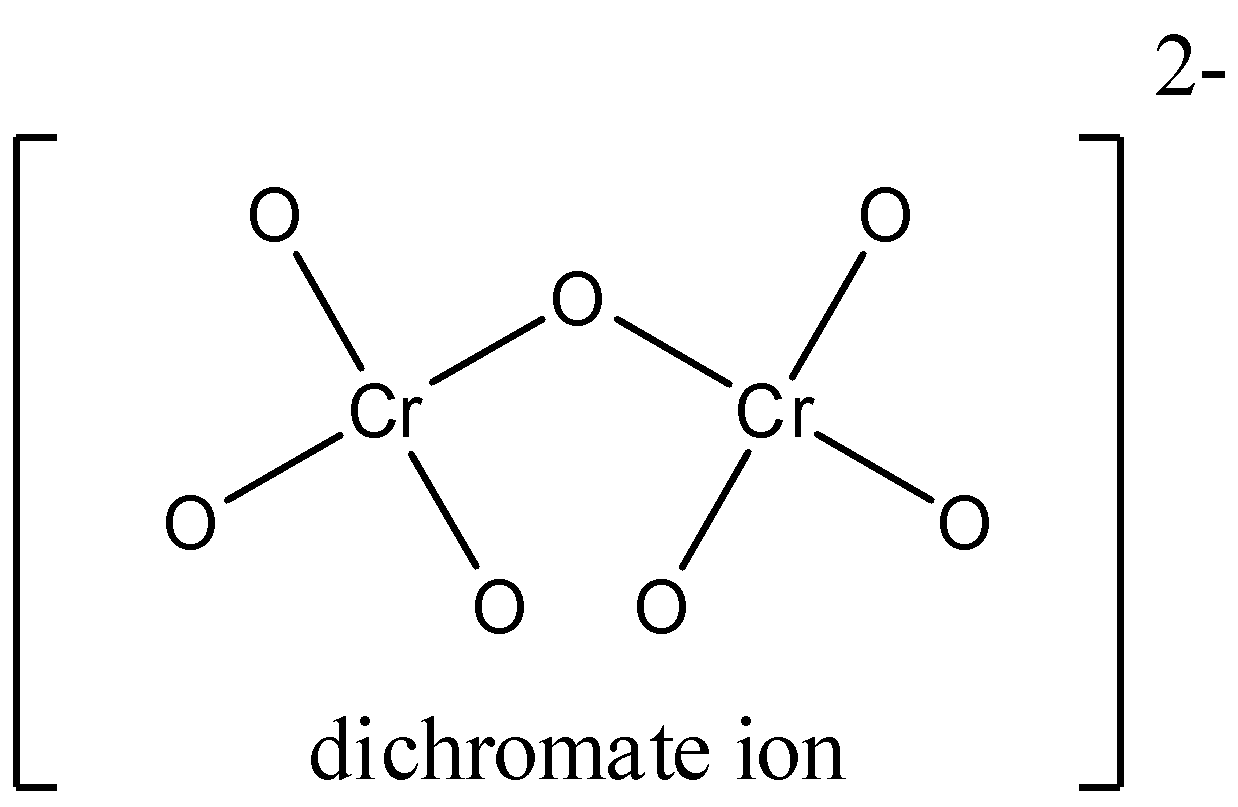
There are eight chromium- oxygen bonds. Among the eight bonds six equivalent chromium- oxygen bonds at terminal.
We know that the Partial double bonds are caused by resonance during a structure, which is the transfer of pi electrons from one site to another. They are said to be partial because the structure can resonate, or flip, between two or more conformations. Of those conformations, a minimum of one will feature a covalent bond between the 2 atoms of interest and a minimum of one will feature one bond.
All the six bonds at terminal end are equivalent due to resonance and these are the oxoanions of chromium and the oxidation state of chromium is $ + 6$.
Therefore, the option C is correct.
Additional Information:
We must know that dichromate anion is a divalent inorganic ion and it is the conjugate base of hydrogen dichromate. Dichromate ion is formed by removing protons from dichromic acid. The molecular formula for dichromic acid is ${H_2}C{r_2}{O_7}$. Dichromate ion is used as an oxidizing agent and it gives many salts because of its ionic property. Both the chromium atoms in dichromate ion present at the center of the tetrahedral bond to four oxygen atoms.
Note:
We can define oxidation state as the degree of loss of an electron in a chemical compound. Now we can calculate the oxidation state of chromium as follows,
Let us take X as the oxidation number of chromium.
The oxidation number of chromium in$\left( {C{r_2}{O_7}^{2 - }} \right)$,
$2X + 7\left( { - 2} \right) = - 2$
$ \Rightarrow 2X - 14 = - 2$
$ \Rightarrow 2X = - 2 + 14$
$ \Rightarrow 2X = + 12$
$ \Rightarrow X = \dfrac{{ + 12}}{2}$
On simplifying we get,
$X = + 6$.
Complete step by step answer:
Now we see the structure of dichromate ion,

There are eight chromium- oxygen bonds. Among the eight bonds six equivalent chromium- oxygen bonds at terminal.
We know that the Partial double bonds are caused by resonance during a structure, which is the transfer of pi electrons from one site to another. They are said to be partial because the structure can resonate, or flip, between two or more conformations. Of those conformations, a minimum of one will feature a covalent bond between the 2 atoms of interest and a minimum of one will feature one bond.
All the six bonds at terminal end are equivalent due to resonance and these are the oxoanions of chromium and the oxidation state of chromium is $ + 6$.
Therefore, the option C is correct.
Additional Information:
We must know that dichromate anion is a divalent inorganic ion and it is the conjugate base of hydrogen dichromate. Dichromate ion is formed by removing protons from dichromic acid. The molecular formula for dichromic acid is ${H_2}C{r_2}{O_7}$. Dichromate ion is used as an oxidizing agent and it gives many salts because of its ionic property. Both the chromium atoms in dichromate ion present at the center of the tetrahedral bond to four oxygen atoms.
Note:
We can define oxidation state as the degree of loss of an electron in a chemical compound. Now we can calculate the oxidation state of chromium as follows,
Let us take X as the oxidation number of chromium.
The oxidation number of chromium in$\left( {C{r_2}{O_7}^{2 - }} \right)$,
$2X + 7\left( { - 2} \right) = - 2$
$ \Rightarrow 2X - 14 = - 2$
$ \Rightarrow 2X = - 2 + 14$
$ \Rightarrow 2X = + 12$
$ \Rightarrow X = \dfrac{{ + 12}}{2}$
On simplifying we get,
$X = + 6$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




