
How nitrobenzene is identified using Mulliken-barker test?
Answer
592.2k+ views
Hint: Mulliken Barker test is used to detect the presence of a mono nitro group in the compound. The presence of the nitro group can be observed by the shiny silver mirror that is formed at the end of the reaction.
Complete answer step by step:
The Mulliken Barker test is based on the fact that the nitro group $(-N{O}_{2})$ is reduced to hydroxylamine group $(-NHOH)$ with the help of a neutral reducing agent. The formed hydroxylamine compound $(-NHOH)$ reduces the Tollen's reagent $([Ag{(N{H}_{3})}_{2}]OH)$ and itself gets oxidised into a nitroso compound.
The following reaction takes place in Mulliken Barker test:
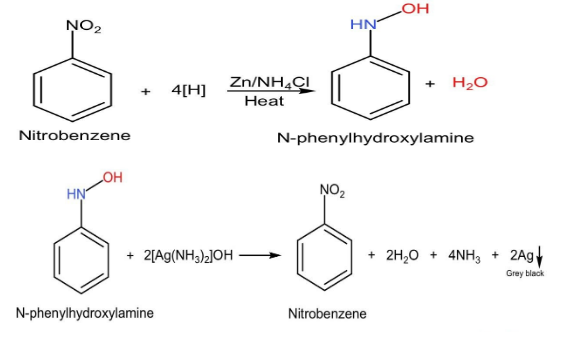
The given nitrobenzene is dissolved in dilute alcohol. To this solution we add a solution of ammonium chloride and zinc dust. Then boil the formed solution for about 2-3 minutes and then filter the solution to another test tube containing the Tollen's reagent. As you pour this solution to the reagent, a grey precipitate is formed and thus will turn to black which will indicate the presence of Nitro group or as we can say it will identify that the compound as nitrobenzene.
Note: This test can be performed for all compounds containing nitro groups and not just nitrobenzene. The Ag released at the end of the reaction indicates the presence of nitro groups in your compound.
Complete answer step by step:
The Mulliken Barker test is based on the fact that the nitro group $(-N{O}_{2})$ is reduced to hydroxylamine group $(-NHOH)$ with the help of a neutral reducing agent. The formed hydroxylamine compound $(-NHOH)$ reduces the Tollen's reagent $([Ag{(N{H}_{3})}_{2}]OH)$ and itself gets oxidised into a nitroso compound.
The following reaction takes place in Mulliken Barker test:

The given nitrobenzene is dissolved in dilute alcohol. To this solution we add a solution of ammonium chloride and zinc dust. Then boil the formed solution for about 2-3 minutes and then filter the solution to another test tube containing the Tollen's reagent. As you pour this solution to the reagent, a grey precipitate is formed and thus will turn to black which will indicate the presence of Nitro group or as we can say it will identify that the compound as nitrobenzene.
Note: This test can be performed for all compounds containing nitro groups and not just nitrobenzene. The Ag released at the end of the reaction indicates the presence of nitro groups in your compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




