
Nitration of benzene is carried out with conc. $\text{HN}{{\text{O}}_{3}}$ in presence of conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$. The role of conc. ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is to provide:
A. nucleophile during the reaction
B. free radical during the reaction
C. electrophile during the reaction
D. catalyst during the reaction
Answer
594k+ views
Hint: The role of ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ in nitration of benzene would be easily understood by looking at the mechanism of nitration on benzene. The product and by-products formed, catalyst involved in the reaction, the type of reaction nitration of benzene is everything could be easily framed with mechanism.
Complete answer:
Nitration of benzene is a type of electrophilic substitution reaction. A substitution reaction in which aromatic compounds hydrogen of benzene nucleus is replaced with a nitro group of concentrated nitric acid in presence of concentrated sulphuric acid which yields nitro derivatives is known as ‘nitration’. The ratio of concentrated nitric acid and concentrated sulphuric acid is (1:2) in the mixture as reagents of the reaction.
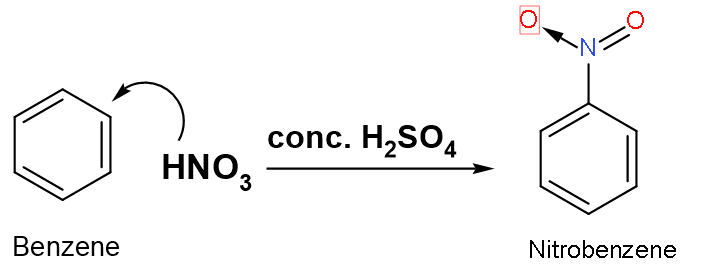
Let us look at the mechanism of the reaction:
Step (1): Formation of electrophile: In nitration, nitronium ion is the electrophile. It is produced by the reaction of nitric acid with sulphuric acid.
$\text{HN}{{\text{O}}_{3}}+\text{2}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\to \overset{+}{\mathop{\text{N}}}\,{{\text{O}}_{2}}+{{\text{H}}_{3}}{{\text{O}}^{+}}+\text{2HS}{{\text{O}}_{4}}^{-}$, where $\overset{+}{\mathop{\text{N}{{\text{O}}_{2}}}}\,$is the electrophile.
Step (2): Formation of carbonium ion: On attacking of electrophile which is$\overset{+}{\mathop{\text{N}{{\text{O}}_{2}}}}\,$on the benzene nucleus, the intermediate carbocation is formed.
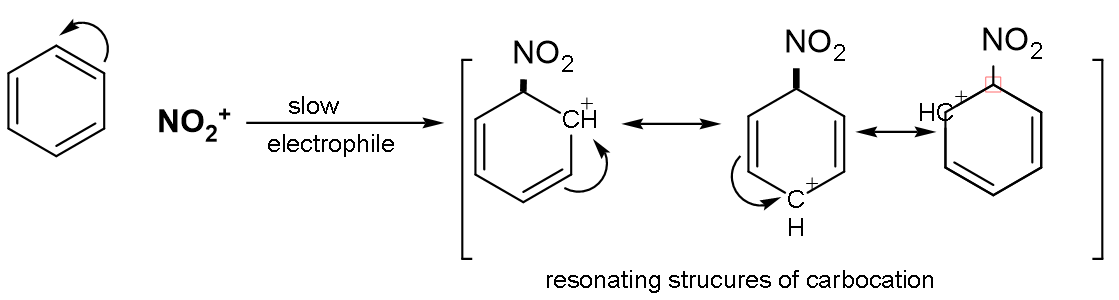
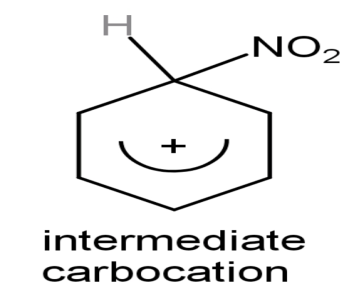
The intermediate carbonium ion is
Step (3): Formation of final product: On attacking the nucleophile that is on the intermediate carbocation and giving the final product by attraction of the proton.
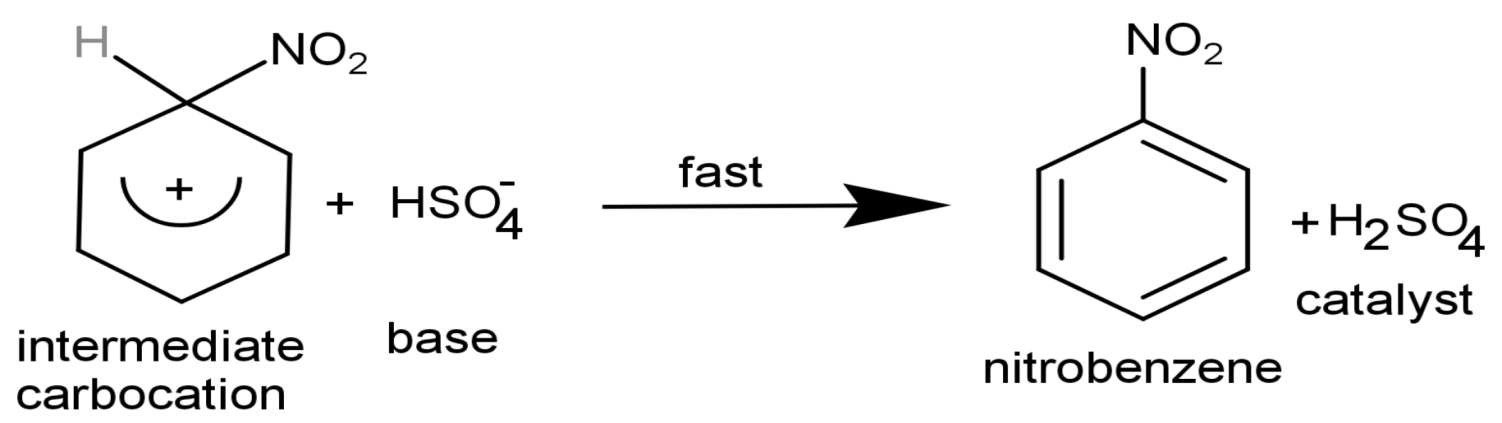
Thus, the concentrated sulphuric acid or ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is acting as a catalyst in nitration reaction with benzene as it is obtained back by the end of the reaction.
So, the correct answer is “Option D”.
Note:
The resonating structures should be made correctly and carefully. The main step is to obtain the electrophile which will be substituted on the benzene. Electrophile does not mean the one with a positive sign on it but the one whose orbitals are empty or electron deficient, like $\text{S}{{\text{O}}_{3}}$. It is an electrophile without having positive charge on its head.
Complete answer:
Nitration of benzene is a type of electrophilic substitution reaction. A substitution reaction in which aromatic compounds hydrogen of benzene nucleus is replaced with a nitro group of concentrated nitric acid in presence of concentrated sulphuric acid which yields nitro derivatives is known as ‘nitration’. The ratio of concentrated nitric acid and concentrated sulphuric acid is (1:2) in the mixture as reagents of the reaction.

Let us look at the mechanism of the reaction:
Step (1): Formation of electrophile: In nitration, nitronium ion is the electrophile. It is produced by the reaction of nitric acid with sulphuric acid.
$\text{HN}{{\text{O}}_{3}}+\text{2}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\to \overset{+}{\mathop{\text{N}}}\,{{\text{O}}_{2}}+{{\text{H}}_{3}}{{\text{O}}^{+}}+\text{2HS}{{\text{O}}_{4}}^{-}$, where $\overset{+}{\mathop{\text{N}{{\text{O}}_{2}}}}\,$is the electrophile.
Step (2): Formation of carbonium ion: On attacking of electrophile which is$\overset{+}{\mathop{\text{N}{{\text{O}}_{2}}}}\,$on the benzene nucleus, the intermediate carbocation is formed.

The intermediate carbonium ion is

Step (3): Formation of final product: On attacking the nucleophile that is on the intermediate carbocation and giving the final product by attraction of the proton.

Thus, the concentrated sulphuric acid or ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is acting as a catalyst in nitration reaction with benzene as it is obtained back by the end of the reaction.
So, the correct answer is “Option D”.
Note:
The resonating structures should be made correctly and carefully. The main step is to obtain the electrophile which will be substituted on the benzene. Electrophile does not mean the one with a positive sign on it but the one whose orbitals are empty or electron deficient, like $\text{S}{{\text{O}}_{3}}$. It is an electrophile without having positive charge on its head.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




