
Nitration of benzene is an example of:
(A) Nucleophilic substitution reaction
(B) Free radical substitution reaction
(C) Electrophilic substitution reaction
(D)Electrophilic addition reaction
Answer
233.1k+ views
Hint: To solve this question we need to know the mechanism of nitration of the benzene ring. The reaction involves treating the benzene ring with conc. Nitric acid along with catalytic conc. Sulphuric acid under specific temperature and pressure conditions which leads to the formation of nitrobenzene.
Complete step by step solution:
To understand which type of reaction nitration of benzene is, we need to look into the mechanism of nitration of benzene. The nitration reaction of benzene is given below:
$ { C }_{ 6 }{ H }_{ 6 }(Benzene)\xrightarrow [ { H }_{ 2 }{ SO }_{ 4 }(Conc.) ]{ { HNO }_{ 3 }(Conc.) } { C }_{ 6 }{ H }_{ 5 }{ (NO }_{ 2 })(Nitrobenzene)$
Let us take a close look at the mechanism of nitration. The first step involves the formation of the nitronium ion. The sulphuric acid is added to nitric acid in order to produce a strong electrophile: nitronium ion. Since sulphuric acid is a stronger acid than nitric acid, it donates its proton to the nitric acid which leads to the formation of nitronium ions. The reaction is shown below:
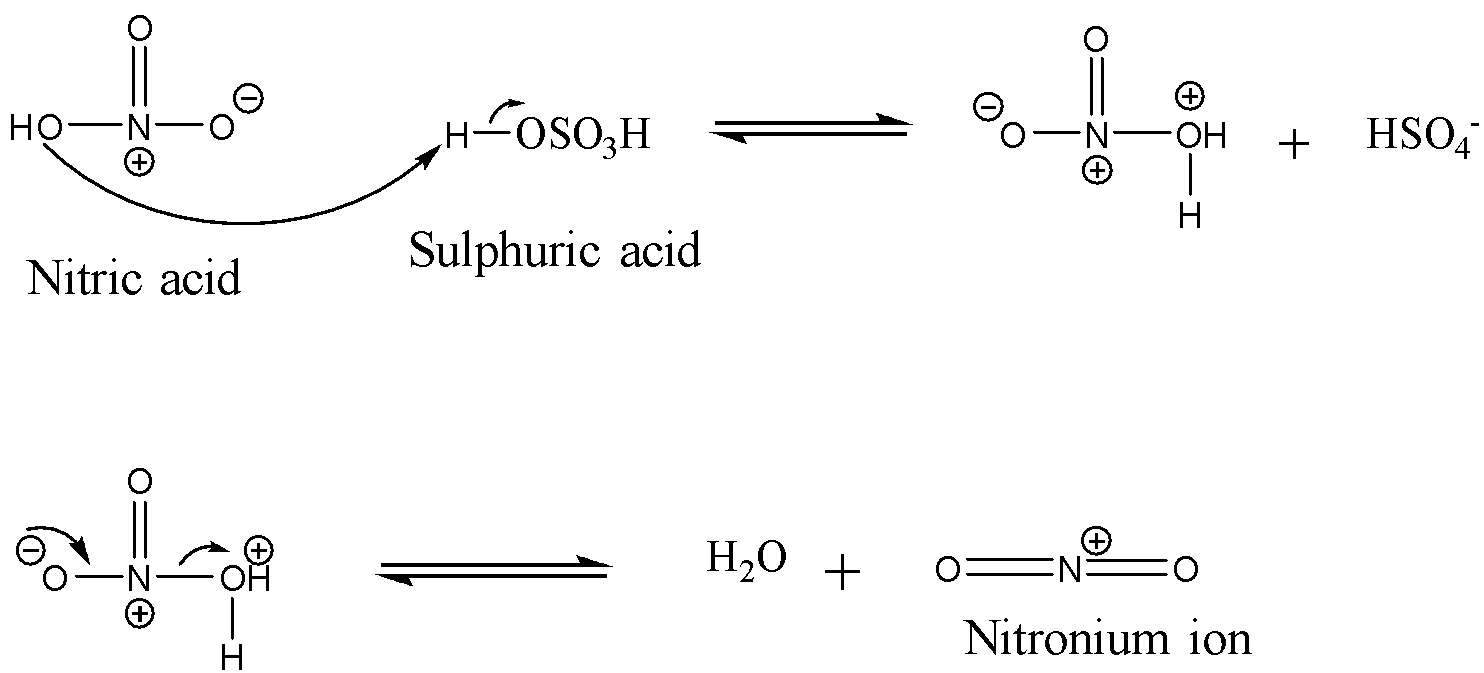
Now, the second step involves the formation of the $\sigma$-complex also called the carbocation intermediate. This happens when the nitronium ion is attacked by the $\pi$ electrons of the benzene ring in order to form an intermediate which is stabilized by resonance. This step is the slowest step and hence is the rate-determining step in this reaction. The reaction is shown below:
The last step involves the loss of a proton from the carbocation intermediate to the base hydrogen sulphate anion to form nitrobenzene. The reaction is shown below: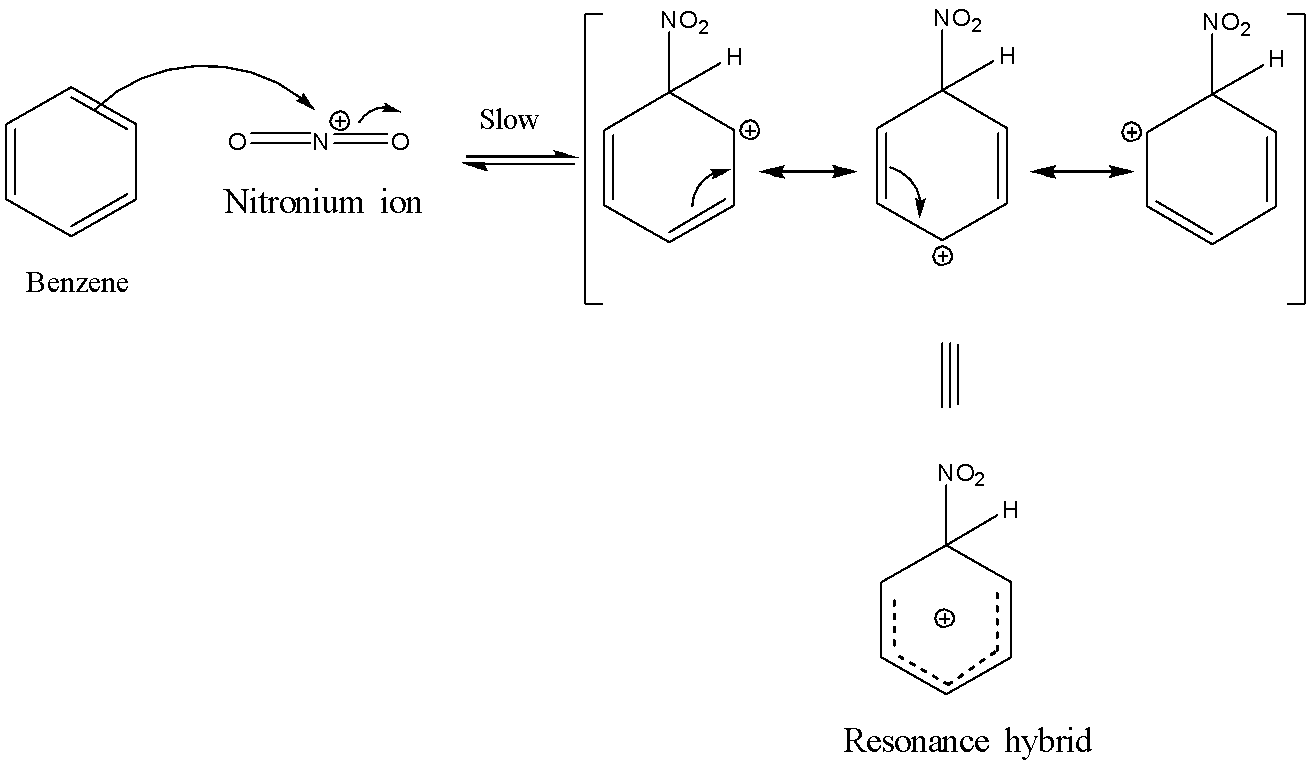
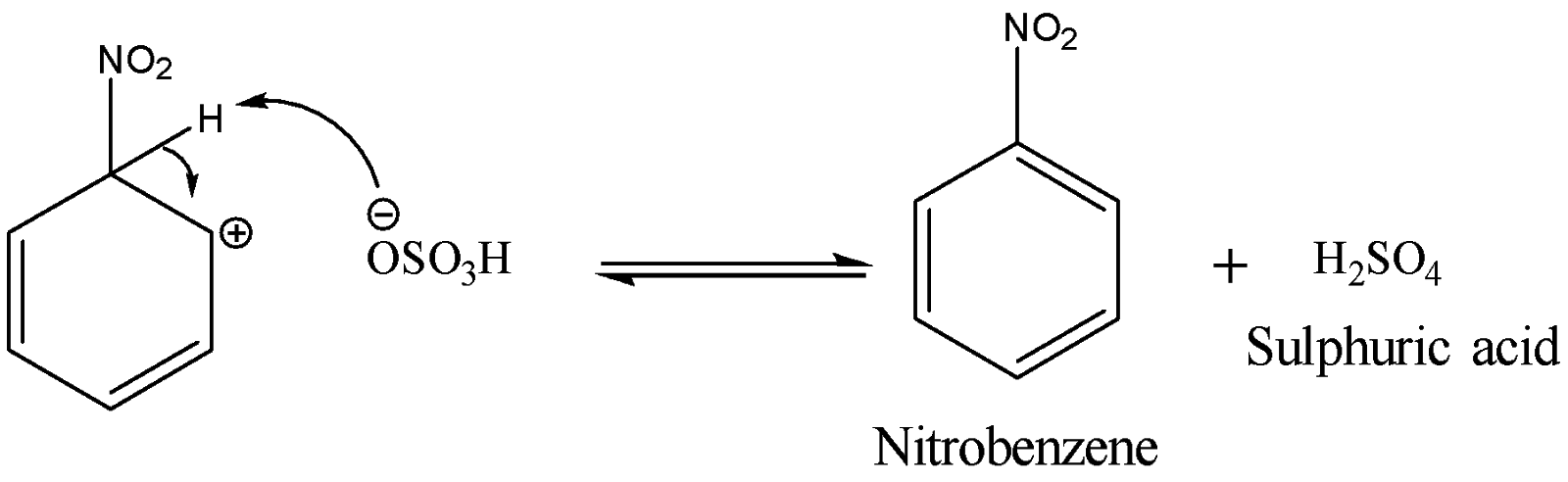
The type of reaction is classified by its rate-determining step. Since this mechanism has a rate-determining step which involves the attack on the nitronium ion which is an electrophile by the benzene ring electrons, therefore nitration of benzene is an electrophilic substitution reaction. It is a substitution reaction because the nitro group is replacing the hydrogen belonging to the benzene.
Hence the correct answer is (C) Electrophilic substitution reaction.
Note: Remember that just like the nitration of benzene is an electrophilic substitution reaction, sulphonation of the benzene is also an electrophilic substitution reaction. In fact, the Friedel-Crafts alkylation; acylation and halogenation of benzene are all electrophilic substitution reactions.
Complete step by step solution:
To understand which type of reaction nitration of benzene is, we need to look into the mechanism of nitration of benzene. The nitration reaction of benzene is given below:
$ { C }_{ 6 }{ H }_{ 6 }(Benzene)\xrightarrow [ { H }_{ 2 }{ SO }_{ 4 }(Conc.) ]{ { HNO }_{ 3 }(Conc.) } { C }_{ 6 }{ H }_{ 5 }{ (NO }_{ 2 })(Nitrobenzene)$
Let us take a close look at the mechanism of nitration. The first step involves the formation of the nitronium ion. The sulphuric acid is added to nitric acid in order to produce a strong electrophile: nitronium ion. Since sulphuric acid is a stronger acid than nitric acid, it donates its proton to the nitric acid which leads to the formation of nitronium ions. The reaction is shown below:

Now, the second step involves the formation of the $\sigma$-complex also called the carbocation intermediate. This happens when the nitronium ion is attacked by the $\pi$ electrons of the benzene ring in order to form an intermediate which is stabilized by resonance. This step is the slowest step and hence is the rate-determining step in this reaction. The reaction is shown below:
The last step involves the loss of a proton from the carbocation intermediate to the base hydrogen sulphate anion to form nitrobenzene. The reaction is shown below:


The type of reaction is classified by its rate-determining step. Since this mechanism has a rate-determining step which involves the attack on the nitronium ion which is an electrophile by the benzene ring electrons, therefore nitration of benzene is an electrophilic substitution reaction. It is a substitution reaction because the nitro group is replacing the hydrogen belonging to the benzene.
Hence the correct answer is (C) Electrophilic substitution reaction.
Note: Remember that just like the nitration of benzene is an electrophilic substitution reaction, sulphonation of the benzene is also an electrophilic substitution reaction. In fact, the Friedel-Crafts alkylation; acylation and halogenation of benzene are all electrophilic substitution reactions.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




