
Neoprene is :
A. Polyester
B. Polyamide
C. Polysaccharide
D. Polyhalodiene
Answer
520.5k+ views
Hint: Neoprene is a synthetic rubber that is flexible over a wide range of temperature that can be found either as solid rubber or in latex form. It is a synthetic polymer that can be used as a rubber alternative and has a large number of applications due to its heat resistance, chemical resistance and flexibility.
Complete answer:
Neoprene is a synthetic homopolymer of chloroprene . Therefore it is also called polychloroprene . It is formed by the free radical polymerization of chloroprene \[(2 - Chloro - buta - 1,3 - diene)\].
Polymerisation is a process in which small repeating molecules called monomers combine chemically to form a larger, chainlike or network molecule called polymer. Additional polymers formed by single monomeric species are called homopolymerization and when two or more different monomeric species are present, then it is called copolymerisation.
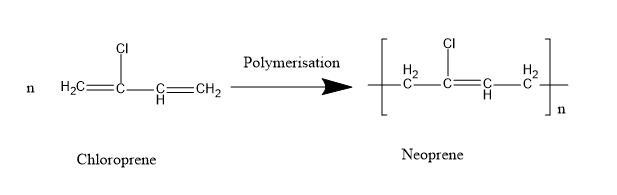
Neoprene is formed by the free radical polymerisation of monomer. Free radical generating initiators like benzoyl peroxide, potassium persulfates are used to initiate the reaction. After the formation of free radicals, the polymer chains are produced.
The monomer chloroprene contains a chlorine atom and two double bonds at first and third carbon atoms. Therefore, the polymer neoprene is polyhalodiene.
The right option is (D) polyhalodiene.
Neoprene is used in manufacture of hoses, conveyor belts, laptop sleeves, remote controls etc. It can also be used to make face masks. Neoprene can resist degradation, corrosion and is waterproof.
Note:
Neoprene can be vulcanised to improve its physical properties like tensile strength. Vulcanisation of rubber is the processing of heating rubber with sulphur and other additives at a high temperature so that sulphur forms cross links at the double bonds. This can stiffen the rubber making it hard and less brittle.
Complete answer:
Neoprene is a synthetic homopolymer of chloroprene . Therefore it is also called polychloroprene . It is formed by the free radical polymerization of chloroprene \[(2 - Chloro - buta - 1,3 - diene)\].
Polymerisation is a process in which small repeating molecules called monomers combine chemically to form a larger, chainlike or network molecule called polymer. Additional polymers formed by single monomeric species are called homopolymerization and when two or more different monomeric species are present, then it is called copolymerisation.

Neoprene is formed by the free radical polymerisation of monomer. Free radical generating initiators like benzoyl peroxide, potassium persulfates are used to initiate the reaction. After the formation of free radicals, the polymer chains are produced.
The monomer chloroprene contains a chlorine atom and two double bonds at first and third carbon atoms. Therefore, the polymer neoprene is polyhalodiene.
The right option is (D) polyhalodiene.
Neoprene is used in manufacture of hoses, conveyor belts, laptop sleeves, remote controls etc. It can also be used to make face masks. Neoprene can resist degradation, corrosion and is waterproof.
Note:
Neoprene can be vulcanised to improve its physical properties like tensile strength. Vulcanisation of rubber is the processing of heating rubber with sulphur and other additives at a high temperature so that sulphur forms cross links at the double bonds. This can stiffen the rubber making it hard and less brittle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




