
Name the reagent used to convert benzyl alcohol to benzoic acid.
Answer
601.8k+ views
Hint: We will need to convert $-C{{H}_{2}}OH$ group of benzyl alcohol into –COOH group to complete this reaction. This is a simple oxidation reaction. An inorganic compound which contains a transition metal in its structure can be used in this conversion.
Complete step by step answer:
- Look, Benzyl alcohol is a primary alcohol and benzoic acid contains a carboxylic acid functional group. So, if we can oxidise the carbon of benzyl alcohol which contains hydroxyl group, then we will get the product benzoic acid.
- We can use an acidic, neutral or alkaline solution of $KMn{{O}_{4}}$ to oxidise any primary alcohol to carboxylic acid. So, we can use alkaline potassium permanganate solution to convert benzyl alcohol to benzoic acid.
- It is proved that out of these types of solutions, acidic potassium permanganate is the most strong reagent. $KMn{{O}_{4}}$ in neutral medium is weaker than it in acidic medium and in when $KMn{{O}_{4}}$ is in alkaline medium, it is weakest amongst three.
- We can write the reaction as follows:
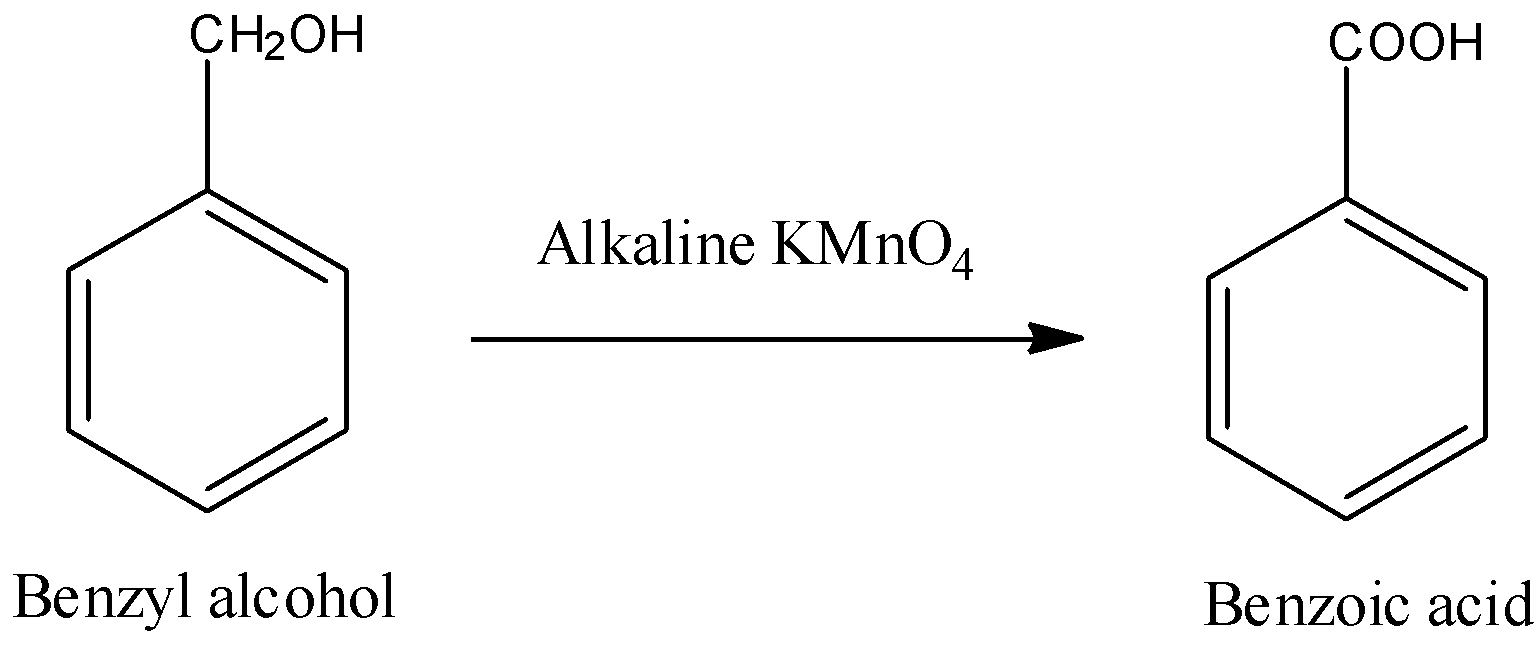
- Remember that Jones reagent ($Cr{{O}_{3}}+{{H}_{2}}S{{O}_{4}}$) can also convert most primary alcohols to carboxylic acids but in case of Benzyl alcohol, Jones reagent will first convert it into Benzaldehyde but this reaction will involve formation of hydrates of aldehyde while, benzaldehyde is not able to form stable hydrates. So, this is the reason that we will not be able to use Jones reagent for this conversion.
Note:
Do not get confused in between benzyl, benzal and benzo prefixes. When ‘benzyl’ prefix is used, it means that the carbon that is attached to the benzene ring has two hydrogen atoms. While in case of benzal, this carbon carries one hydrogen atom and in case of benzo, there is no hydrogen attached to the carbon that is directly bonded to the benzene ring.
Complete step by step answer:
- Look, Benzyl alcohol is a primary alcohol and benzoic acid contains a carboxylic acid functional group. So, if we can oxidise the carbon of benzyl alcohol which contains hydroxyl group, then we will get the product benzoic acid.
- We can use an acidic, neutral or alkaline solution of $KMn{{O}_{4}}$ to oxidise any primary alcohol to carboxylic acid. So, we can use alkaline potassium permanganate solution to convert benzyl alcohol to benzoic acid.
- It is proved that out of these types of solutions, acidic potassium permanganate is the most strong reagent. $KMn{{O}_{4}}$ in neutral medium is weaker than it in acidic medium and in when $KMn{{O}_{4}}$ is in alkaline medium, it is weakest amongst three.
- We can write the reaction as follows:

- Remember that Jones reagent ($Cr{{O}_{3}}+{{H}_{2}}S{{O}_{4}}$) can also convert most primary alcohols to carboxylic acids but in case of Benzyl alcohol, Jones reagent will first convert it into Benzaldehyde but this reaction will involve formation of hydrates of aldehyde while, benzaldehyde is not able to form stable hydrates. So, this is the reason that we will not be able to use Jones reagent for this conversion.
Note:
Do not get confused in between benzyl, benzal and benzo prefixes. When ‘benzyl’ prefix is used, it means that the carbon that is attached to the benzene ring has two hydrogen atoms. While in case of benzal, this carbon carries one hydrogen atom and in case of benzo, there is no hydrogen attached to the carbon that is directly bonded to the benzene ring.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




