
Name the monomer present in the following polymer
A. Polyvinyl chloride
B. Natural rubber.
Answer
564.9k+ views
Hint: Many compounds show different properties when treated with different reagents and go through different reactions. Some of the compounds tend to form a chain of the compound with a repeating structure. This can be termed as polymerization. During this process, a compound reacts and attaches to another molecule of the same compound to form a chain structure and hence form what is called a polymer.
Complete step by step answer:
Polymerization is the process of the monomer reacting to form a repeated chain which contains itself in a long chain compound. Usually, it takes thousands of monomers to form a polymer. Monomers are the single compounds that react with the other molecule of its type and form a repeated chain of the same compound and thus make polymers.
Polyvinyl chloride is the polymer that is made up of the compound of vinyl chloride as a monomer.
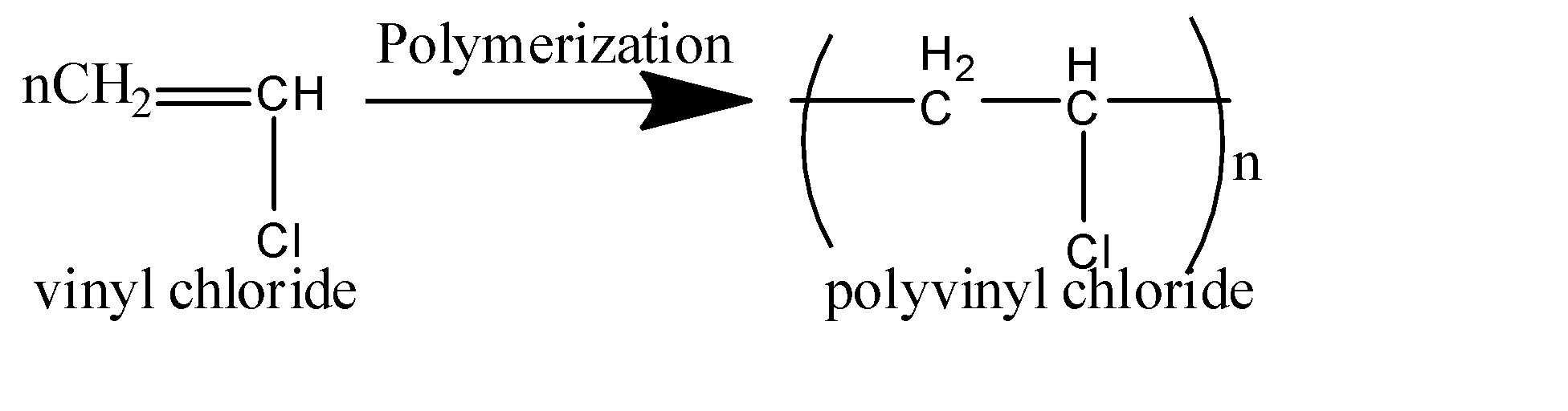
Vinyl chloride combines to form a long chain of the molecule and thus forms polyvinyl chloride.
Polyisoprene is the compound that is used for the manufacture of natural rubber. It polymerizes to form the natural rubber structure by repeating in the large chain.
Polyisoprene can also be written as 2-methyl-1,3-butadiene. The polymer is formed as a milky white fluid which can be converted as latex for the manufacture of commercial rubber.
Note: PVC is a commercially useful polymer and is used for the manufacture of pipes and cables etc., PVC in some places is a substitute for the rubber and plastic used. Natural rubber is made from latex which is extracted from plants and then refined and processed to get the final form of commercially viable rubber.
Complete step by step answer:
Polymerization is the process of the monomer reacting to form a repeated chain which contains itself in a long chain compound. Usually, it takes thousands of monomers to form a polymer. Monomers are the single compounds that react with the other molecule of its type and form a repeated chain of the same compound and thus make polymers.
Polyvinyl chloride is the polymer that is made up of the compound of vinyl chloride as a monomer.

Vinyl chloride combines to form a long chain of the molecule and thus forms polyvinyl chloride.
Polyisoprene is the compound that is used for the manufacture of natural rubber. It polymerizes to form the natural rubber structure by repeating in the large chain.
Polyisoprene can also be written as 2-methyl-1,3-butadiene. The polymer is formed as a milky white fluid which can be converted as latex for the manufacture of commercial rubber.
Note: PVC is a commercially useful polymer and is used for the manufacture of pipes and cables etc., PVC in some places is a substitute for the rubber and plastic used. Natural rubber is made from latex which is extracted from plants and then refined and processed to get the final form of commercially viable rubber.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




