
Name the constituents of a cell.
Answer
583.2k+ views
Hint: The electric cells are devices which produce electric DC current by chemical reactions. There are various types of cells and each has its own chemical and structural composition. There are also similarities in them despite the differences, which make them a cell.
Complete answer:
Any electric cell works on the cathode – anode reaction between two electrically opposite materials. In every cell, there is a cathode, an anode and an electrolyte. Let us discuss the various cells and find out the composition in them.
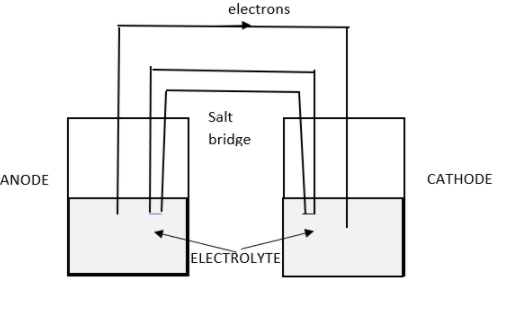
1) Primary cell: These are also known as dry cells or galvanic cells. They are the basic cells which we use in toys, remote controllers, clocks and other stuffs. They are non – rechargeable once their energy runs out. The composition of the cell is:
a) Anode: Zinc electrode – reduction reaction takes place
b) Cathode: Copper electrode – oxidation takes place.
c) Electrolyte: copper sulphate and zinc sulphate
d) Salt bridge: Balances the charge concentration in the total cell.
2) Secondary cell: These are rechargeable cells used in automobiles and inverters. These can be charged and discharged repeatedly for a long time. There are various combinations of anode cathode that are used as storage batteries like Pb – acid, Ni – Cd, Li- ion and so on. Let us discuss the most widely used Lead – acid cell.
a) The cell undergoes reverse reactions in charging and discharging modes.
b) The hydrogen ion in the acid helps the lead rods placed in the sulphuric acid to oxide to lead sulphate during discharge.
c) The lead gets deposited during charging which compensates the metal consumed and thus recharging guarantees future use.
d) The total reaction involved is:
\[\begin{align}
& Pb(s)+Pb{{O}_{2}}(s)+2{{H}_{2}}S{{O}_{4}}(aq)\leftrightarrow \text{ }2PbS{{O}_{4}}(s)+2{{H}_{2}}O(l) \\
& {{E}^{0}}_{cell}=2.05V \\
\end{align}\]
These are the main components in most of the cells. Depending on the combination of anode – cathode used they vary in chemical reaction and efficiency.
Note:
Any cell whether rechargeable or not, requires replacement and proper maintenance for efficiency. The battery water added to the automobiles batteries at regular intervals compensates the acid which get reduced due to other reactions like corrosion of metal parts.
A Hydrogen cell is considered a zero-pollutant as its by-product is just water.
Complete answer:
Any electric cell works on the cathode – anode reaction between two electrically opposite materials. In every cell, there is a cathode, an anode and an electrolyte. Let us discuss the various cells and find out the composition in them.

1) Primary cell: These are also known as dry cells or galvanic cells. They are the basic cells which we use in toys, remote controllers, clocks and other stuffs. They are non – rechargeable once their energy runs out. The composition of the cell is:
a) Anode: Zinc electrode – reduction reaction takes place
b) Cathode: Copper electrode – oxidation takes place.
c) Electrolyte: copper sulphate and zinc sulphate
d) Salt bridge: Balances the charge concentration in the total cell.
2) Secondary cell: These are rechargeable cells used in automobiles and inverters. These can be charged and discharged repeatedly for a long time. There are various combinations of anode cathode that are used as storage batteries like Pb – acid, Ni – Cd, Li- ion and so on. Let us discuss the most widely used Lead – acid cell.
a) The cell undergoes reverse reactions in charging and discharging modes.
b) The hydrogen ion in the acid helps the lead rods placed in the sulphuric acid to oxide to lead sulphate during discharge.
c) The lead gets deposited during charging which compensates the metal consumed and thus recharging guarantees future use.
d) The total reaction involved is:
\[\begin{align}
& Pb(s)+Pb{{O}_{2}}(s)+2{{H}_{2}}S{{O}_{4}}(aq)\leftrightarrow \text{ }2PbS{{O}_{4}}(s)+2{{H}_{2}}O(l) \\
& {{E}^{0}}_{cell}=2.05V \\
\end{align}\]
These are the main components in most of the cells. Depending on the combination of anode – cathode used they vary in chemical reaction and efficiency.
Note:
Any cell whether rechargeable or not, requires replacement and proper maintenance for efficiency. The battery water added to the automobiles batteries at regular intervals compensates the acid which get reduced due to other reactions like corrosion of metal parts.
A Hydrogen cell is considered a zero-pollutant as its by-product is just water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




