
How do you name alkane from Newman projections?
Answer
552.3k+ views
Hint: Count carbon atoms which constitute the maximum number, note that newman projections are only drawn for alkane it means only for saturated hydrocarbons where there is only one sigma bond and at least two carbons constitutes in this projection. Count the maximum number of carbons and then termed them according to their number, ethane, propane, butane…. for $2,\,3,\,4........$ carbons atoms.
Complete step-by-step answer:
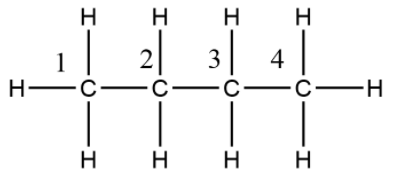
The above system is of n-butane having four carbon atoms, so here if we give numbering to all carbon atoms as $1,\,2,\,3,\,4$ the for converting them in newman projection we take carbon $2$ and carbon $3$ to represent by a dot and a circle, other two carbons of methyl group are taken as their bonds.
To define a system with respect to their structure in $3 - Dimension$ , there are various theories given to do this work. Newman projection is one of them, there are fisher, flying wedge, saw horse etc. In these types of structural representation, scientists always gave an approach to take the maximum number of carbon atoms on the straight line. Lets see firstly how a newman projection looks like,
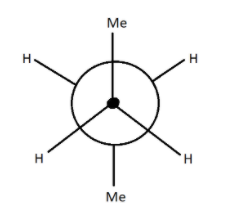
Here the figure shows newman structure, in this projection the carbon facing us, that carbon which is towards us is represented by a dot while the carbon which is at the back side will be shown by a circle. Now imagine that the hydrogen carbon is held like you are seeing it as a three dimensional figure but for drawing it on a paper, it will look like as shown by the figure. So, here are two carbon holds like this one towards us and the other one shown by a circle.
There is two methyl groups at ${180^ \circ }$ to each other and other hydrogen atoms are also ${180^ \circ }$, now it is always not like same that all hydrogens are at ${180^ \circ }$ of angle but the bulky groups should be at maximum distance to each other so that both feel lesser amount of repulsion of each other electron cloud. If we count two methyl, there will now be four carbon atoms for which butane name is given according to IUPAC. Counting the maximum number of carbon atoms is our priority while considering a Newman projection. So, the above compound is n-butane in which two methyl are at ${180^ \circ }$ of angle to reduce repulsion.
Note: Make two carbon as a dot and circle and try to adjust other groups in such a way that they must have maximum distance from each other and lesser repulsion. The two methyl groups are connected with carbon $2$ and $3$ so you have to connect the methyl groups with carbon $2$ and $3$.
Complete step-by-step answer:

The above system is of n-butane having four carbon atoms, so here if we give numbering to all carbon atoms as $1,\,2,\,3,\,4$ the for converting them in newman projection we take carbon $2$ and carbon $3$ to represent by a dot and a circle, other two carbons of methyl group are taken as their bonds.
To define a system with respect to their structure in $3 - Dimension$ , there are various theories given to do this work. Newman projection is one of them, there are fisher, flying wedge, saw horse etc. In these types of structural representation, scientists always gave an approach to take the maximum number of carbon atoms on the straight line. Lets see firstly how a newman projection looks like,

Here the figure shows newman structure, in this projection the carbon facing us, that carbon which is towards us is represented by a dot while the carbon which is at the back side will be shown by a circle. Now imagine that the hydrogen carbon is held like you are seeing it as a three dimensional figure but for drawing it on a paper, it will look like as shown by the figure. So, here are two carbon holds like this one towards us and the other one shown by a circle.
There is two methyl groups at ${180^ \circ }$ to each other and other hydrogen atoms are also ${180^ \circ }$, now it is always not like same that all hydrogens are at ${180^ \circ }$ of angle but the bulky groups should be at maximum distance to each other so that both feel lesser amount of repulsion of each other electron cloud. If we count two methyl, there will now be four carbon atoms for which butane name is given according to IUPAC. Counting the maximum number of carbon atoms is our priority while considering a Newman projection. So, the above compound is n-butane in which two methyl are at ${180^ \circ }$ of angle to reduce repulsion.
Note: Make two carbon as a dot and circle and try to adjust other groups in such a way that they must have maximum distance from each other and lesser repulsion. The two methyl groups are connected with carbon $2$ and $3$ so you have to connect the methyl groups with carbon $2$ and $3$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




