
Mononitration of phenyl methanoate.
Answer
532.8k+ views
Hint: Formate whose IUPAC name is methanoate is the anion derived from formic acid. Its formula is chloroformate, $C{H_3}OCOCl$. The combined influence of —$OH$ and —$C{H_3}$ groups determines the position of the incoming group.
Complete answer:
Phenyl methanoate is a compound having very less shelf- life. So, it is very unstable. Its formula is ${C_6}{H_5}OCOH$.
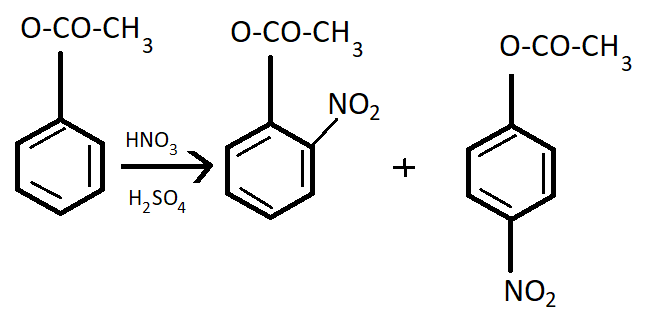
It goes to the para position because the Benzene ring will be unstable if the $N{O_2}$group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
This process occurs in the presence of $HN{O_3}$ and ${H_2}S{O_4}$which is the nitrating mixture.
Note: Phenyl methanoate is a compound having very less life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a meta product. In such types of problems, the properties of compounds are very important.
Complete answer:
Phenyl methanoate is a compound having very less shelf- life. So, it is very unstable. Its formula is ${C_6}{H_5}OCOH$.

It goes to the para position because the Benzene ring will be unstable if the $N{O_2}$group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
This process occurs in the presence of $HN{O_3}$ and ${H_2}S{O_4}$which is the nitrating mixture.
Note: Phenyl methanoate is a compound having very less life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a meta product. In such types of problems, the properties of compounds are very important.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




