
Methoxy benzene on treatment with cold HI produces
A. Iodobenzene and methanol
B. Phenol and methyl iodide
C. Iodobenzene and methyl iodide
D. Phenol and methanol.
Answer
592.8k+ views
Hint: Anisole is the other name for methoxybenzene. When methoxy benzene and hydrogen iodide react together and the bond between oxygen and methyl reacts, it breaks down. The bond between oxygen and benzene does not break because due to resonance it has a partial double bond and hence stronger.
Complete answer:
-When methoxy benzene is treated with cold hydrogen iodide then the bond present between oxygen and methyl group is methoxy group breaks down.
-This leads to the formation of phenoxide ion with the release of methyl ion.
-Now, the hydrogen iodide breaks into hydrogen ion and iodide ion.
-The hydrogen ion now attaches with the oxygen present in the phenoxide ion and forms phenol which have a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
-The chemical reaction is:
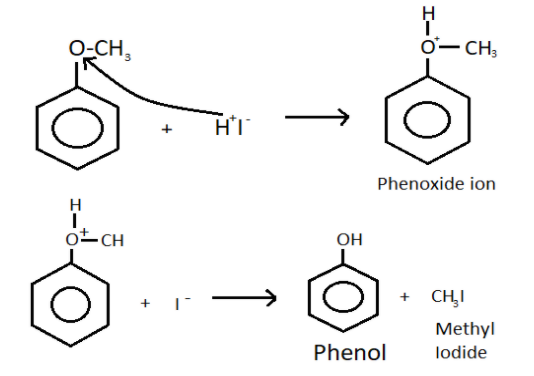
-Here, the bond formed between oxygen and methyl of the methoxy group is a weaker bond than the bond present between the oxygen and benzene ring.
-Because due to resonance, the partial double bond is present between the carbon of the benzene ring and oxygen.
-Along with the phenol, methyl iodide is also formed because methyl ion was released from the methoxy group and iodine was released by hydrogen iodide, they combine and form methyl iodide.
So, the correct answer is “Option B”.
Note: Resonance is a phenomenon in which the delocalisation of π- bond electrons takes place. Resonance structure having more stability should have a greater number of the covalent bond and least formal charge.
Complete answer:
-When methoxy benzene is treated with cold hydrogen iodide then the bond present between oxygen and methyl group is methoxy group breaks down.
-This leads to the formation of phenoxide ion with the release of methyl ion.
-Now, the hydrogen iodide breaks into hydrogen ion and iodide ion.
-The hydrogen ion now attaches with the oxygen present in the phenoxide ion and forms phenol which have a molecular formula of ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
-The chemical reaction is:

-Here, the bond formed between oxygen and methyl of the methoxy group is a weaker bond than the bond present between the oxygen and benzene ring.
-Because due to resonance, the partial double bond is present between the carbon of the benzene ring and oxygen.
-Along with the phenol, methyl iodide is also formed because methyl ion was released from the methoxy group and iodine was released by hydrogen iodide, they combine and form methyl iodide.
So, the correct answer is “Option B”.
Note: Resonance is a phenomenon in which the delocalisation of π- bond electrons takes place. Resonance structure having more stability should have a greater number of the covalent bond and least formal charge.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




