
What is the major product formed when methylene cyclohexane reacts with NBS?
Answer
573.6k+ views
Hint: Understand the reagent called NBS. Determine the group taking part in the reaction along with understanding the reaction mechanism. Perform the reaction on the reactant, methylene cyclohexane and thereby determine the product as well as answer the question.
Complete Solution :
- NBS stands for N-Bromosuccinimide is a chemical reagent used mainly in radical substitution, electrophilic addition as well as in electrophilic substitution reactions in organic chemistry.
- It is considered as a convenient source of the bromine radical.
- In alkenes, NBS reacts with the compound in an aqueous solution to give bromohydrins as the product.
- We will draw the expanded structure of methylene cyclohexane and then perform the reaction with the reagent, NBS.
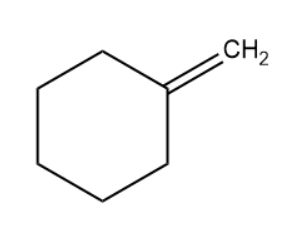
- Allylic bromination is considered as the reaction using NBS leading to the replacement of hydrogens on a carbon adjacent to a double bond with bromine atom.
- The reaction explained above will now be performed on methylene cyclohexane to determine the product.
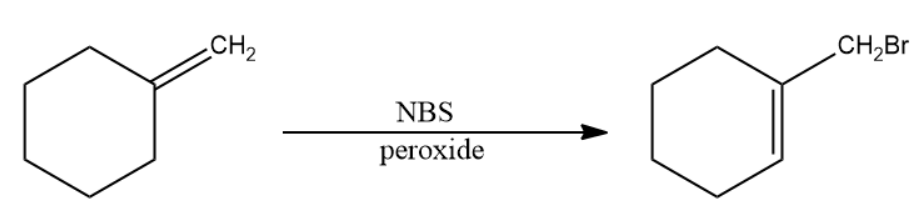
The IUPAC name of the product is 1-(bromomethyl)cyclohexene.
So, the correct answer is “Option C”.
Note: In the above reaction, we see that along with NBS, a peroxide is used usually. This is because peroxide is used as a radical initiator. NBS on its own cannot produce bromine radicals. Peroxide helps in the homolytic fission of the N-Br bond and generates bromine free radicals.
Complete Solution :
- NBS stands for N-Bromosuccinimide is a chemical reagent used mainly in radical substitution, electrophilic addition as well as in electrophilic substitution reactions in organic chemistry.
- It is considered as a convenient source of the bromine radical.
- In alkenes, NBS reacts with the compound in an aqueous solution to give bromohydrins as the product.
- We will draw the expanded structure of methylene cyclohexane and then perform the reaction with the reagent, NBS.

- Allylic bromination is considered as the reaction using NBS leading to the replacement of hydrogens on a carbon adjacent to a double bond with bromine atom.
- The reaction explained above will now be performed on methylene cyclohexane to determine the product.

The IUPAC name of the product is 1-(bromomethyl)cyclohexene.
So, the correct answer is “Option C”.
Note: In the above reaction, we see that along with NBS, a peroxide is used usually. This is because peroxide is used as a radical initiator. NBS on its own cannot produce bromine radicals. Peroxide helps in the homolytic fission of the N-Br bond and generates bromine free radicals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




