
What is the major product formed when methylene cyclohexane reacts with NBS?
Answer
582.6k+ views
Hint: Understand the reagent called NBS. Determine the group taking part in the reaction along with understanding the reaction mechanism. Perform the reaction on the reactant, methylene cyclohexane and thereby determine the product as well as answer the question.
Complete Solution :
- NBS stands for N-Bromosuccinimide is a chemical reagent used mainly in radical substitution, electrophilic addition as well as in electrophilic substitution reactions in organic chemistry.
- It is considered as a convenient source of the bromine radical.
- In alkenes, NBS reacts with the compound in an aqueous solution to give bromohydrins as the product.
- We will draw the expanded structure of methylene cyclohexane and then perform the reaction with the reagent, NBS.
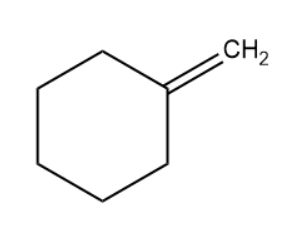
- Allylic bromination is considered as the reaction using NBS leading to the replacement of hydrogens on a carbon adjacent to a double bond with bromine atom.
- The reaction explained above will now be performed on methylene cyclohexane to determine the product.
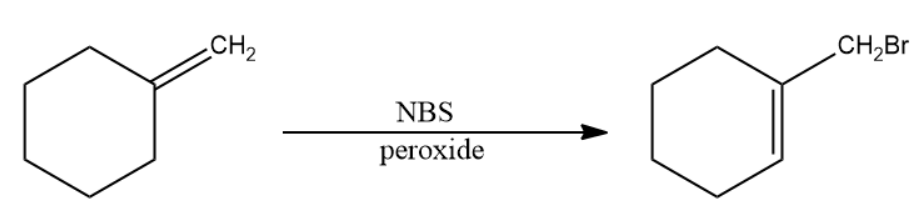
The IUPAC name of the product is 1-(bromomethyl)cyclohexene.
So, the correct answer is “Option C”.
Note: In the above reaction, we see that along with NBS, a peroxide is used usually. This is because peroxide is used as a radical initiator. NBS on its own cannot produce bromine radicals. Peroxide helps in the homolytic fission of the N-Br bond and generates bromine free radicals.
Complete Solution :
- NBS stands for N-Bromosuccinimide is a chemical reagent used mainly in radical substitution, electrophilic addition as well as in electrophilic substitution reactions in organic chemistry.
- It is considered as a convenient source of the bromine radical.
- In alkenes, NBS reacts with the compound in an aqueous solution to give bromohydrins as the product.
- We will draw the expanded structure of methylene cyclohexane and then perform the reaction with the reagent, NBS.

- Allylic bromination is considered as the reaction using NBS leading to the replacement of hydrogens on a carbon adjacent to a double bond with bromine atom.
- The reaction explained above will now be performed on methylene cyclohexane to determine the product.

The IUPAC name of the product is 1-(bromomethyl)cyclohexene.
So, the correct answer is “Option C”.
Note: In the above reaction, we see that along with NBS, a peroxide is used usually. This is because peroxide is used as a radical initiator. NBS on its own cannot produce bromine radicals. Peroxide helps in the homolytic fission of the N-Br bond and generates bromine free radicals.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




