
It is difficult to break \[\text{C - Cl}\] bond in \[\text{C}{{\text{H}}_{2}}\text{ = CHCl}\] due to:
A. Hyperconjugation
B. Resonance
C. Electromeric effect
D. Inductive effect
Answer
573.6k+ views
Hint: The pi - bond is responsible for the delocalisation in the structures whereas sigma bond cannot do so as sigma bond is localised. The delocalisation of the pi - bond electrons can make the partial double bond which is stronger than the sigma bond.
Complete Step-by-Step Answer:
- In the given question, we have to tell why it is difficult to break the sigma bond present between carbon and a chlorine atom.
- As we know that resonance is the phenomenon in which the delocalization of the pi-bond takes place.
- The structures formed by the action of resonance are known as canonical structures or resonance hybrid structures.
- In the given structure, the resonance structure will be:
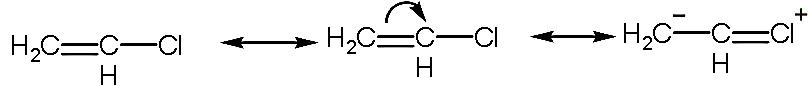
- So, there are three canonical structures of chloroethene.
- Due to the resonance the partial double bond is formed between the carbon and chlorine atom which is stronger than the sigma or single bond.
- Whereas hyperconjugation is delocalisation of the lone pair electrons with the adjacent pi-bond.
- It is less strong than the resonance because, in resonance, $\pi \text{-}\pi \text{ bond}$ delocalisation take place whereas in hyperconjugation, $\sigma \text{-}\pi \text{ bond}$ delocalisation takes place.
- Electromeric effect is a transfer of a pi-bond electron to another atom with the help of any reagent either nucleophile or electrophile.
- Whereas in inductive effect the electrons are attracted by the more electronegative element. Due to which the partial charge is formed on the elements in resonance complete transfer of electron takes place.
Therefore, option B is the correct answer.
Note: In resonance, the most electronegative atom has a negative charge whereas the electropositive atom has the positive charge. More is the resonance structure or canonical structure less will be the energy of the molecule and more it will be stable.
Complete Step-by-Step Answer:
- In the given question, we have to tell why it is difficult to break the sigma bond present between carbon and a chlorine atom.
- As we know that resonance is the phenomenon in which the delocalization of the pi-bond takes place.
- The structures formed by the action of resonance are known as canonical structures or resonance hybrid structures.
- In the given structure, the resonance structure will be:

- So, there are three canonical structures of chloroethene.
- Due to the resonance the partial double bond is formed between the carbon and chlorine atom which is stronger than the sigma or single bond.
- Whereas hyperconjugation is delocalisation of the lone pair electrons with the adjacent pi-bond.
- It is less strong than the resonance because, in resonance, $\pi \text{-}\pi \text{ bond}$ delocalisation take place whereas in hyperconjugation, $\sigma \text{-}\pi \text{ bond}$ delocalisation takes place.
- Electromeric effect is a transfer of a pi-bond electron to another atom with the help of any reagent either nucleophile or electrophile.
- Whereas in inductive effect the electrons are attracted by the more electronegative element. Due to which the partial charge is formed on the elements in resonance complete transfer of electron takes place.
Therefore, option B is the correct answer.
Note: In resonance, the most electronegative atom has a negative charge whereas the electropositive atom has the positive charge. More is the resonance structure or canonical structure less will be the energy of the molecule and more it will be stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




