
In which of the following arrangements, a metal would have the least density?
(A) BCC
(B)CCP
(C)HCP
(D)SC
Answer
583.2k+ views
Hint: Solids that have definite shape and volume. It has definite shape and volume because the constituent molecules are arranged in particular a pattern from which it cannot move. The arrangement of the unit cell that has least packing efficiency will have the least density.
Complete step by step answer:
Solids have definite shape and volume because of the arrangement of the constituent molecule which cannot move from its place but can vibrate about its mean position. The number of structural arrangements of constituent particles as a point in the three dimensional structure and the number of lattice points together forms a unit cell. The number unit collectively forms a crystal.
The total number of atoms per unit cell and packing efficiency differs from one type of arrangement to the other type of arrangement. The arrangement that has least packing efficiency will have the least density.
Option A is "BBC". The full form of "BBC" is a body centered cubic unit cell. The unit cell in BBC has an atom at its each corner and one atom at its center. The total number of atoms per unit cell is 2 atoms. The packing efficiency of a body centered cubic unit cell is 68%.
Option B is "CCP". The full form is cubic close packing. The arrangement of spheres (lattice) in the cubic structure where all the spheres touch each other. Its packing efficiency is 74%.
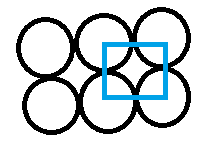
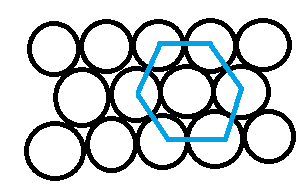
Option C is "HCP". The full form is hexagonal closed structure. The spheres touch in such a way that 5 voids are created (diagram mentioned below). Its packing efficiency is 74%.
Option D is " SC". The full form of SC is simple cubic. The number atom in a unit cell is 1. The packing efficiency of SC is 52.4%
Therefore, option D is the correct answer as the arrangement which has a low packing efficiency has the least density.
Note: Comparing the packing efficiency of all options, the SC has lowest packing efficiency by 52.4%. It is very important to remember the number of atoms per unit cell and the packing efficiency in hand because it is used frequently to solve problems in higher studies.
Complete step by step answer:
Solids have definite shape and volume because of the arrangement of the constituent molecule which cannot move from its place but can vibrate about its mean position. The number of structural arrangements of constituent particles as a point in the three dimensional structure and the number of lattice points together forms a unit cell. The number unit collectively forms a crystal.
The total number of atoms per unit cell and packing efficiency differs from one type of arrangement to the other type of arrangement. The arrangement that has least packing efficiency will have the least density.
Option A is "BBC". The full form of "BBC" is a body centered cubic unit cell. The unit cell in BBC has an atom at its each corner and one atom at its center. The total number of atoms per unit cell is 2 atoms. The packing efficiency of a body centered cubic unit cell is 68%.
Option B is "CCP". The full form is cubic close packing. The arrangement of spheres (lattice) in the cubic structure where all the spheres touch each other. Its packing efficiency is 74%.

Option C is "HCP". The full form is hexagonal closed structure. The spheres touch in such a way that 5 voids are created (diagram mentioned below). Its packing efficiency is 74%.

Option D is " SC". The full form of SC is simple cubic. The number atom in a unit cell is 1. The packing efficiency of SC is 52.4%
Therefore, option D is the correct answer as the arrangement which has a low packing efficiency has the least density.
Note: Comparing the packing efficiency of all options, the SC has lowest packing efficiency by 52.4%. It is very important to remember the number of atoms per unit cell and the packing efficiency in hand because it is used frequently to solve problems in higher studies.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




