
In the spinel structure, oxides ions are cubically-closed packed whereas ${{\dfrac{1}{8}}^{th}}$of tetrahedral voids are occupied by ${{A}^{2+}}$cation and half of octahedral voids are occupied by ${{B}^{3+}}$cations. The general formula of compound having spinel structure is:
(1) ${{A}_{2}}{{B}_{2}}{{O}_{4}}$
(2) $A{{B}_{2}}{{O}_{4}}$
(3) ${{A}_{2}}{{B}_{4}}{{O}_{2}}$
(4) ${{A}_{4}}{{B}_{2}}{{O}_{2}}$
Answer
583.2k+ views
Hint: Generally, tetrahedral voids are two times octahedral voids in cubic close packed structures.
Complete step by step solution:
Let us first understand about the packed structures and voids present within them.
Cubic close packed structures-
The cubic close packed structure consists of four layers of atoms per unit cell. There are two types of arrangement of atoms in the structure i.e. Simple cubic arrangement and Hexagonal cubic arrangement.
Simple cubic arrangement has the same layer of atoms arranged layer by layer one above the other in a definite manner.
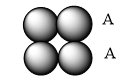
Hexagonal cubic arrangement has one layer in the depression of another layer, repeating over one another.
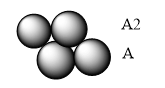
There are two types of gaps (commonly known as voids) within them;
Tetrahedral void-
These are mostly found in CCP and are triangular in shape with coordination number of 4.
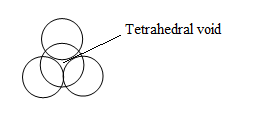
Tetrahedral voids are two times the number of atoms in the unit cell.
Octahedral void-
These are mostly found in HCP and are octahedral in shape with coordination number of 6.
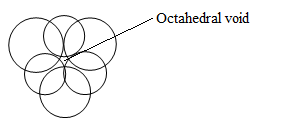
Octahedral voids are equal to the number of atoms in the unit cell.
Thus,
Tetrahedral voids are two times the octahedral voids.
Spinel group-
These are the group of minerals with general formulation $A{{B}_{2}}{{X}_{4}}$.
where,
X is anion
A and B are cations.
Illustration,
Oxides form close packings. Thus, it is an anion.
Whereas, ${{A}^{2+}}$ and ${{B}^{3+}}$ are cations.
Given that,
${{\dfrac{1}{8}}^{th}}$of tetrahedral voids are occupied by ${{A}^{2+}}$cation;
Thus, ${{A}^{2+}}$ ions will be ${{\dfrac{1}{4}}^{th}}$of oxide ions.
And half of octahedral voids are occupied by ${{B}^{3+}}$cations;
Thus, ${{B}^{3+}}$ ions will be $\dfrac{1}{2}$ of oxide ions.
Hence, A:B:O = $\dfrac{1}{4}$: $\dfrac{1}{2}$: 1 = 1:2:4.
Therefore, the formula is $A{{B}_{2}}{{O}_{4}}$.
Option (B) is correct.
Note: In accordance with the hint given, we can ignore option (A) and (D) from the start. Only concentrate on the remaining two options.
Complete step by step solution:
Let us first understand about the packed structures and voids present within them.
Cubic close packed structures-
The cubic close packed structure consists of four layers of atoms per unit cell. There are two types of arrangement of atoms in the structure i.e. Simple cubic arrangement and Hexagonal cubic arrangement.
Simple cubic arrangement has the same layer of atoms arranged layer by layer one above the other in a definite manner.

Hexagonal cubic arrangement has one layer in the depression of another layer, repeating over one another.

There are two types of gaps (commonly known as voids) within them;
Tetrahedral void-
These are mostly found in CCP and are triangular in shape with coordination number of 4.

Tetrahedral voids are two times the number of atoms in the unit cell.
Octahedral void-
These are mostly found in HCP and are octahedral in shape with coordination number of 6.

Octahedral voids are equal to the number of atoms in the unit cell.
Thus,
Tetrahedral voids are two times the octahedral voids.
Spinel group-
These are the group of minerals with general formulation $A{{B}_{2}}{{X}_{4}}$.
where,
X is anion
A and B are cations.
Illustration,
Oxides form close packings. Thus, it is an anion.
Whereas, ${{A}^{2+}}$ and ${{B}^{3+}}$ are cations.
Given that,
${{\dfrac{1}{8}}^{th}}$of tetrahedral voids are occupied by ${{A}^{2+}}$cation;
Thus, ${{A}^{2+}}$ ions will be ${{\dfrac{1}{4}}^{th}}$of oxide ions.
And half of octahedral voids are occupied by ${{B}^{3+}}$cations;
Thus, ${{B}^{3+}}$ ions will be $\dfrac{1}{2}$ of oxide ions.
Hence, A:B:O = $\dfrac{1}{4}$: $\dfrac{1}{2}$: 1 = 1:2:4.
Therefore, the formula is $A{{B}_{2}}{{O}_{4}}$.
Option (B) is correct.
Note: In accordance with the hint given, we can ignore option (A) and (D) from the start. Only concentrate on the remaining two options.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

How many states of matter are there in total class 12 chemistry CBSE

What are the advantages of vegetative propagation class 12 biology CBSE

Suicide bags of cells are aEndoplasmic reticulum bLysosome class 12 biology CBSE

What is the Full Form of PVC, PET, HDPE, LDPE, PP and PS ?




