
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is :
a.) formation of carbocation
b.) formation of an ester
c.) protonation of alcohol molecule
d.) elimination of water
Answer
592.8k+ views
Hint: In the first step of dehydration of alcohol to an alkene, the atom with lone pair i.e. the oxygen atom will attack the proton abstracting it. The sulphuric acid can donate two protons which are attached with two oxygen atoms.
Complete answer:
First, let us see dehydration of alcohols to alkenes by heating with concentrated sulphuric acid. The word dehydration means the removal of water. So, there will be a loss of water in the reaction.
The sulphuric acid is present as -
${H_2}S{O_4} \rightleftharpoons {H^ + } + HSO_4^ - $
Mechanism of dehydration of alcohols to alkenes :
In the first step, the oxygen atom of alcohol attacks the ${H^ + }$ ion of sulphuric acid. This results in the formation of alkyloxonium ion which has a positive charge on oxygen and oxygen are bonded to three atoms.
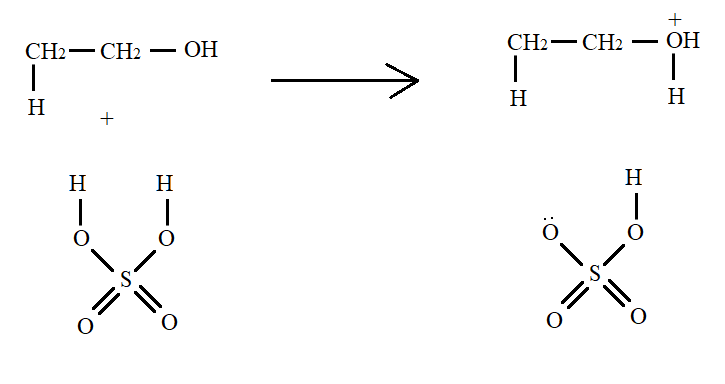
Such a molecule is not so stable. The lone pair in the oxygen of sulphuric acid abstracts a proton from the molecule. This results in breaking of carbon-oxygen bond and formation of alkene along with a molecule of water.
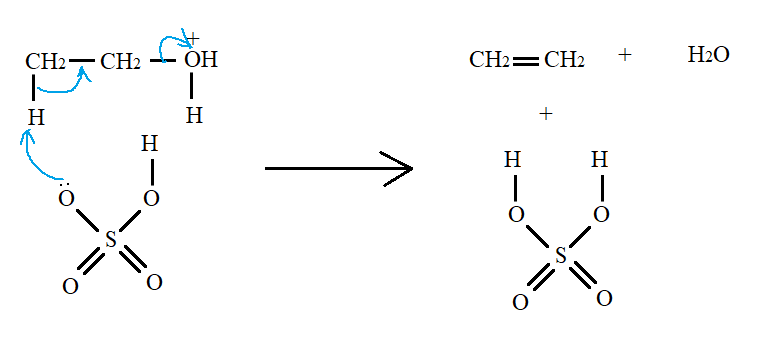
Thus, the initiation step is the protonation of the alcohol molecule.
So, the correct answer is “Option C”.
Note: The positive charge on oxygen in the first step is not so stable because oxygen is a highly electronegative and positive charge on an electronegative molecule is not so stable.
Complete answer:
First, let us see dehydration of alcohols to alkenes by heating with concentrated sulphuric acid. The word dehydration means the removal of water. So, there will be a loss of water in the reaction.
The sulphuric acid is present as -
${H_2}S{O_4} \rightleftharpoons {H^ + } + HSO_4^ - $
Mechanism of dehydration of alcohols to alkenes :
In the first step, the oxygen atom of alcohol attacks the ${H^ + }$ ion of sulphuric acid. This results in the formation of alkyloxonium ion which has a positive charge on oxygen and oxygen are bonded to three atoms.

Such a molecule is not so stable. The lone pair in the oxygen of sulphuric acid abstracts a proton from the molecule. This results in breaking of carbon-oxygen bond and formation of alkene along with a molecule of water.

Thus, the initiation step is the protonation of the alcohol molecule.
So, the correct answer is “Option C”.
Note: The positive charge on oxygen in the first step is not so stable because oxygen is a highly electronegative and positive charge on an electronegative molecule is not so stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




