
In the complex ion${{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}$
A. Fe is in the +1 oxidation state, and NO coordinates as $N{{O}^{+}}$ (nitrosonium ion)
B. Fe is in the +2 oxidation state, and NO coordinates as neutral NO (nitrosyl) radical
C. Fe is in the +3 oxidation state and NO coordinates as $N{{O}^{-}}$
D. Fe is in the +2 oxidation state and NO coordinates as $N{{O}^{+}}$
Answer
573.3k+ views
Hint: In order to find the oxidation number of iron, the oxidation numbers for ${{H}_{2}}O$ and NO need to be known. Then an equation can be formed, which on solving would give the required oxidation number for iron.
Complete answer:
In order to answer our question, we need to learn about the coordination compounds. Now, when a salt is dissolved in water, it dissociates into ions, for example, NaCl dissociates into $N{{a}^{+}}$and $C{{l}^{-}}$ions. A double salt is the complex form of the salt which has more entities in it, for example, Mohr’s salt. Coordination compounds also have many entities attached to the central metal atom, but they do not dissociate into ions, dissolving in water. Coordination may be defined as the number of coordinate bonds formed with central atom/ion the ligands. For example in coordination entity ${{[Ag{{(CN)}_{2}}]}^{-}},{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}},{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}$, the coordination number of Ag, Cu and Cr are 2, 4 and 6 respectively. Similarly in ${{[Fe{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\,and\,{{[Co{{(en)}_{3}}]}^{3+}}$ the coordination number of both Fe and Co is 6 because ${{C}_{2}}{{O}_{4}}^{2-}$(oxalate) and en (ethylene-1, 2-diamine) forms two coordinate bonds each with central metal atom/ion.
Oxidation number: Oxidation number of the central metal atom ion in a complex is the charge present on it if all the ligands are removed along with the electron pairs that are shared with the central atom. It is represented by Roman numerals in parentheses after the name of central atom For example, oxidation number of Co Fe and Ni in ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Fe{{(CN)}_{6}}]}^{4-}}\,and\,[Ni{{(CO)}_{4}}]$ is +3, +2 and 0, and written as Co(i), Fe(ii) and Ni(0) respectively. The compound looks like:
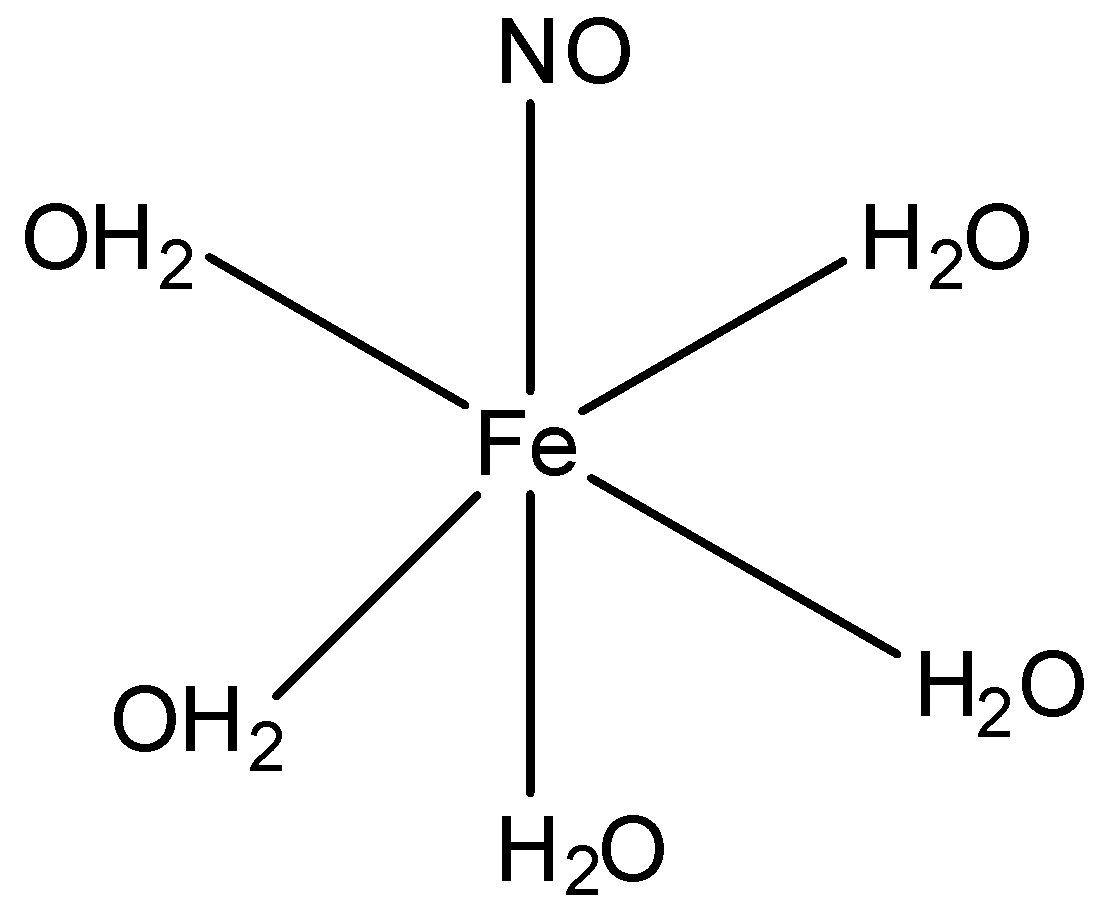
Let the oxidation number of iron be ‘x’. Now, we have:$x+(5\times 0)+0=2$,as NO and ${{H}_{2}}O$have oxidation number 0. Also the compound is in+2 state. So, x comes out to be +2 and NO is present as nitrosyl radical.
So our correct answer would be option B.
NOTE: It is not necessary that the oxidation number we find out has to be positive. Oxidation numbers can be positive, negative as well as 0. Oxidation number cannot be a fraction, however if it comes as a fraction, then the rule is not valid. In such cases, the structure of the compound will help to determine the real oxidation state.
Complete answer:
In order to answer our question, we need to learn about the coordination compounds. Now, when a salt is dissolved in water, it dissociates into ions, for example, NaCl dissociates into $N{{a}^{+}}$and $C{{l}^{-}}$ions. A double salt is the complex form of the salt which has more entities in it, for example, Mohr’s salt. Coordination compounds also have many entities attached to the central metal atom, but they do not dissociate into ions, dissolving in water. Coordination may be defined as the number of coordinate bonds formed with central atom/ion the ligands. For example in coordination entity ${{[Ag{{(CN)}_{2}}]}^{-}},{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}},{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}$, the coordination number of Ag, Cu and Cr are 2, 4 and 6 respectively. Similarly in ${{[Fe{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\,and\,{{[Co{{(en)}_{3}}]}^{3+}}$ the coordination number of both Fe and Co is 6 because ${{C}_{2}}{{O}_{4}}^{2-}$(oxalate) and en (ethylene-1, 2-diamine) forms two coordinate bonds each with central metal atom/ion.
Oxidation number: Oxidation number of the central metal atom ion in a complex is the charge present on it if all the ligands are removed along with the electron pairs that are shared with the central atom. It is represented by Roman numerals in parentheses after the name of central atom For example, oxidation number of Co Fe and Ni in ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Fe{{(CN)}_{6}}]}^{4-}}\,and\,[Ni{{(CO)}_{4}}]$ is +3, +2 and 0, and written as Co(i), Fe(ii) and Ni(0) respectively. The compound looks like:

Let the oxidation number of iron be ‘x’. Now, we have:$x+(5\times 0)+0=2$,as NO and ${{H}_{2}}O$have oxidation number 0. Also the compound is in+2 state. So, x comes out to be +2 and NO is present as nitrosyl radical.
So our correct answer would be option B.
NOTE: It is not necessary that the oxidation number we find out has to be positive. Oxidation numbers can be positive, negative as well as 0. Oxidation number cannot be a fraction, however if it comes as a fraction, then the rule is not valid. In such cases, the structure of the compound will help to determine the real oxidation state.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




