
In ${{{C}}_{{3}}}^{{{4 - }}}$, the number of ${{\sigma }}$- and ${{\pi }}$- bonds present between carbon atoms are:
A.${{1\sigma and 1\pi }}$
B.${{2\sigma and 2\pi }}$
C.${{2\sigma and 1\pi }}$
D.${{1\sigma }}$ bond only
Answer
565.5k+ views
Hint:Sigma bond is formed by the head-on overlap of atomic orbitals. It is the strongest covalent bond. It is formed by the overlap of orbitals in an end to end fashion. There are different types of sigma bonds which are ${{s - s}}$ , ${{s - p}}$ and ${{p - p}}$ . This is an example of a p-p sigma bond. When 2 lobes of an atomic orbital overlap on the orbital of another atom, it is a bond or ${{\pi bond}}$. The Sigma bond is shown by the symbol $\sigma {{ }}$ and the bond by the symbol ${{\pi }}$ .
Complete step by step answer:
We know that carbon has 4 valence electrons.
In this question, there are 3 carbon atom
So the parent chain can be drawn as $C - C - C$
The charge on the ${{{C}}_{{3}}}$ is -4.
Let us take the middle carbon. We know that carbon has a valency of 4, thus it is attached to 2 carbon atoms with 4 bonds.
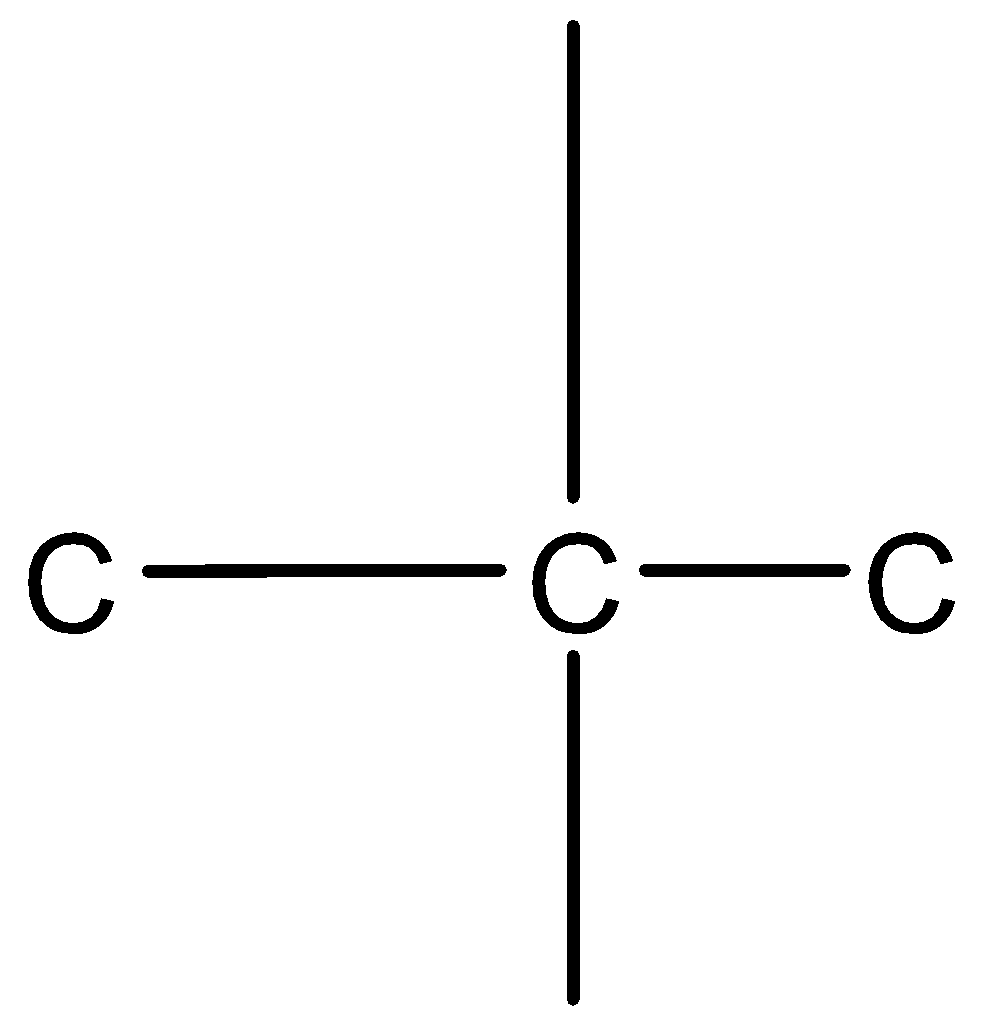
Since the middle carbon is attached to only 2 carbon atoms, its 2 valencies are shared with the adjacent two carbon atoms.
Now, there is no hydrogen atom and the molecule has a negative charge.
So, the remaining 2 lone valence electrons undergo lateral overlap forming 2 ${{\pi bonds}}$.
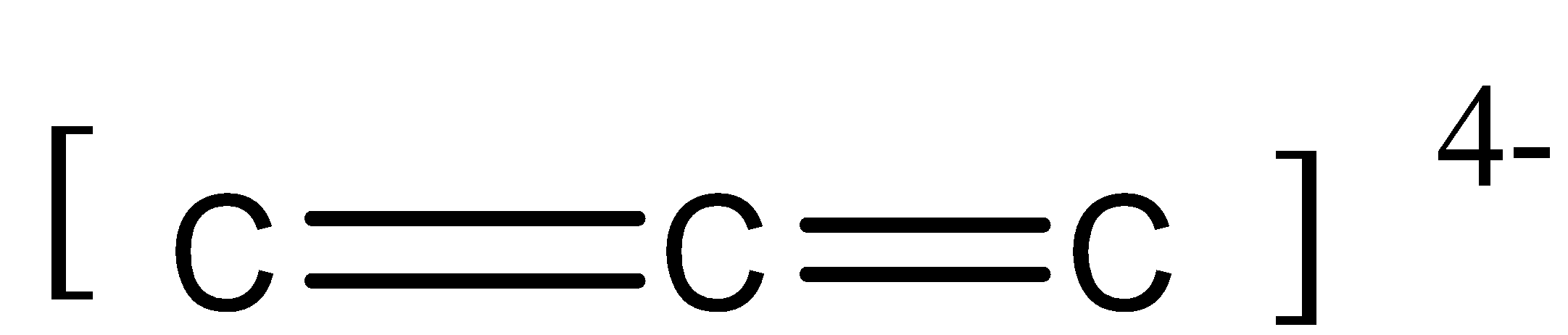
There is an overall $ - 4$ charge. It is due to the non-bonded 2 electrons on the first and third carbon atom.
Thus we can say that in this molecule, there is 2 sigma bond and 2 pi bond ($2\sigma {{ bond}}$ and ${{2\pi bond}}$).
The correct answer is an option (B).
Note:
When an example of hydrocarbon is taken, carbon with all ${{\sigma }}$ bonds is ${{s}}{{{p}}^{{3}}}$ hybridized. The carbon with 1 $\sigma {{ bond}}$ and 1 $\pi $ bond forms a ${{s}}{{{p}}^{{2}}}$ hybridized and if the carbon is attached to 2 $\pi $ bonds, then it is sp hybridized.

Complete step by step answer:
We know that carbon has 4 valence electrons.
In this question, there are 3 carbon atom
So the parent chain can be drawn as $C - C - C$
The charge on the ${{{C}}_{{3}}}$ is -4.
Let us take the middle carbon. We know that carbon has a valency of 4, thus it is attached to 2 carbon atoms with 4 bonds.

Since the middle carbon is attached to only 2 carbon atoms, its 2 valencies are shared with the adjacent two carbon atoms.
Now, there is no hydrogen atom and the molecule has a negative charge.
So, the remaining 2 lone valence electrons undergo lateral overlap forming 2 ${{\pi bonds}}$.
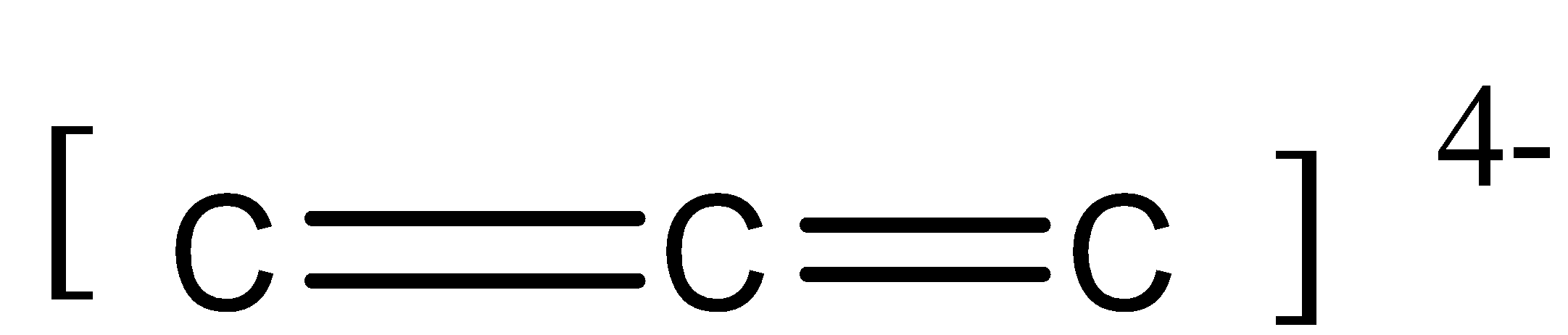
There is an overall $ - 4$ charge. It is due to the non-bonded 2 electrons on the first and third carbon atom.
Thus we can say that in this molecule, there is 2 sigma bond and 2 pi bond ($2\sigma {{ bond}}$ and ${{2\pi bond}}$).
The correct answer is an option (B).
Note:
When an example of hydrocarbon is taken, carbon with all ${{\sigma }}$ bonds is ${{s}}{{{p}}^{{3}}}$ hybridized. The carbon with 1 $\sigma {{ bond}}$ and 1 $\pi $ bond forms a ${{s}}{{{p}}^{{2}}}$ hybridized and if the carbon is attached to 2 $\pi $ bonds, then it is sp hybridized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




