
In a dry cell………………… acts as a positive terminal.
A) Carbon rod
B) Manganese dioxide
C) Manganese dioxide and powered carbon
D) Metal cap on the carbon rod
Answer
594k+ views
Hint:A dry cell is a type of electric battery; it is a portable electrical device. A dry cell is a zinc-carbon cell. It consists of one or more electrochemical cells. Dry cell can operate without spilling, redox reaction takes place as it consists of fluid, it converts chemical energy into electrical energy. Ammonium chloride is the electrolyte used in dry cells.
Complete step by step answer:
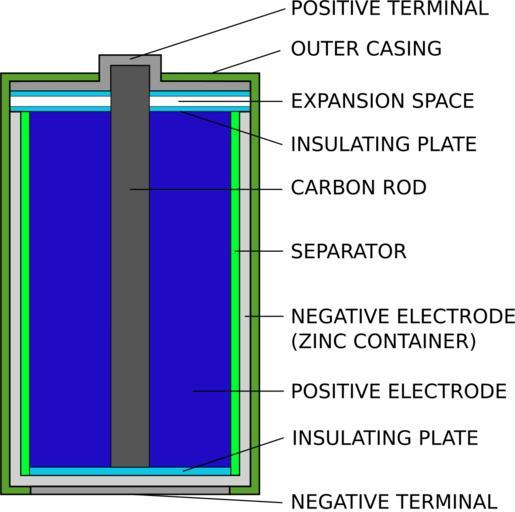
Let us consider the given diagram of a dry cell. There is a part called a zinc container. This zinc container acts as a negative electrode. We can see a carbon rod part. This carbon rod forms a positive electrode. The carbon rod is coated with $Mn{O_2}$ and powdered carbon. The powdered carbon will reduce the internal resistance of the cell and hence the metal rod on the carbon rod also acts as a positive terminal.
According to the nature of the dry cell, it can be divided into two types, primary cell and the secondary cell. A battery and the dry cell are not the same, a battery contains electrochemical cells that store chemical energy and convert the chemical energy to electrical energy. The carbon or manganese dioxide reacts with zinc, this chemical reaction produces the electricity in the dry cell. When a dry cell is connected in a circuit and a bulb is connected to it, due to the chemical reaction the current flows in the circuit that makes the bulb glow.
Primary dry cell: It is neither reusable nor rechargeable and these can be handled for a long period of storage as they lose their charges very slowly.
Secondary dry cell: It is rechargeable by using battery charges, to regenerate the chemical reactions.
The correct option is (D).
Note:1. Use of dry cell: Dry cells are more durable therefore these are used in remote control devices, flashlights, etc.
2. Dry cells are environment friendly, therefore nowadays most automobiles come equipped with dry cells.
3. Dry cells are comparatively lighter in weight.
4. Wet cells use Sulfuric acid as an electrolyte which is corrosive to the environment.
Complete step by step answer:

Let us consider the given diagram of a dry cell. There is a part called a zinc container. This zinc container acts as a negative electrode. We can see a carbon rod part. This carbon rod forms a positive electrode. The carbon rod is coated with $Mn{O_2}$ and powdered carbon. The powdered carbon will reduce the internal resistance of the cell and hence the metal rod on the carbon rod also acts as a positive terminal.
According to the nature of the dry cell, it can be divided into two types, primary cell and the secondary cell. A battery and the dry cell are not the same, a battery contains electrochemical cells that store chemical energy and convert the chemical energy to electrical energy. The carbon or manganese dioxide reacts with zinc, this chemical reaction produces the electricity in the dry cell. When a dry cell is connected in a circuit and a bulb is connected to it, due to the chemical reaction the current flows in the circuit that makes the bulb glow.
Primary dry cell: It is neither reusable nor rechargeable and these can be handled for a long period of storage as they lose their charges very slowly.
Secondary dry cell: It is rechargeable by using battery charges, to regenerate the chemical reactions.
The correct option is (D).
Note:1. Use of dry cell: Dry cells are more durable therefore these are used in remote control devices, flashlights, etc.
2. Dry cells are environment friendly, therefore nowadays most automobiles come equipped with dry cells.
3. Dry cells are comparatively lighter in weight.
4. Wet cells use Sulfuric acid as an electrolyte which is corrosive to the environment.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




