
In 18- crown-6, the number of an oxygen atom is:
A. 18
B. 6
C. 12
D. 24
Answer
579k+ views
Hint: The main concept behind solving this question is 18-Crown-6. First we need to know that 18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride.
Complete step by step answer:
We know that 18-Crown-6 has the ability to solubilize metal salts, and particularly potassium salts, in non-polar and dipolar aprotic solvents. So, it is widely used as a phase transfer catalyst. We can also use it as a metal complex agent in the preparation of a variety of molecular complexes. It is also used as phase-transfer catalysts.
The chemical formula of the organic compound 18-Crown-6 is ${[{C_2}{H_4}O]_6}$ and the IUPAC name is 1, 4, 7, 10, 13, 16-hexaoxacyclooctadecane. This organic compound is found as a white, hygroscopic crystalline solid with a low melting point.
So, to derive the number of an oxygen atom in a crown ether 18-crown-6 is 6. It is represented by the number of an oxygen atom and here, 18 represents the sum of the total number of carbon and oxygen atoms in the ether. Therefore, the number of an oxygen atom in 18-crown-6 is 6.
We can write the Structure of 18-crown-6:
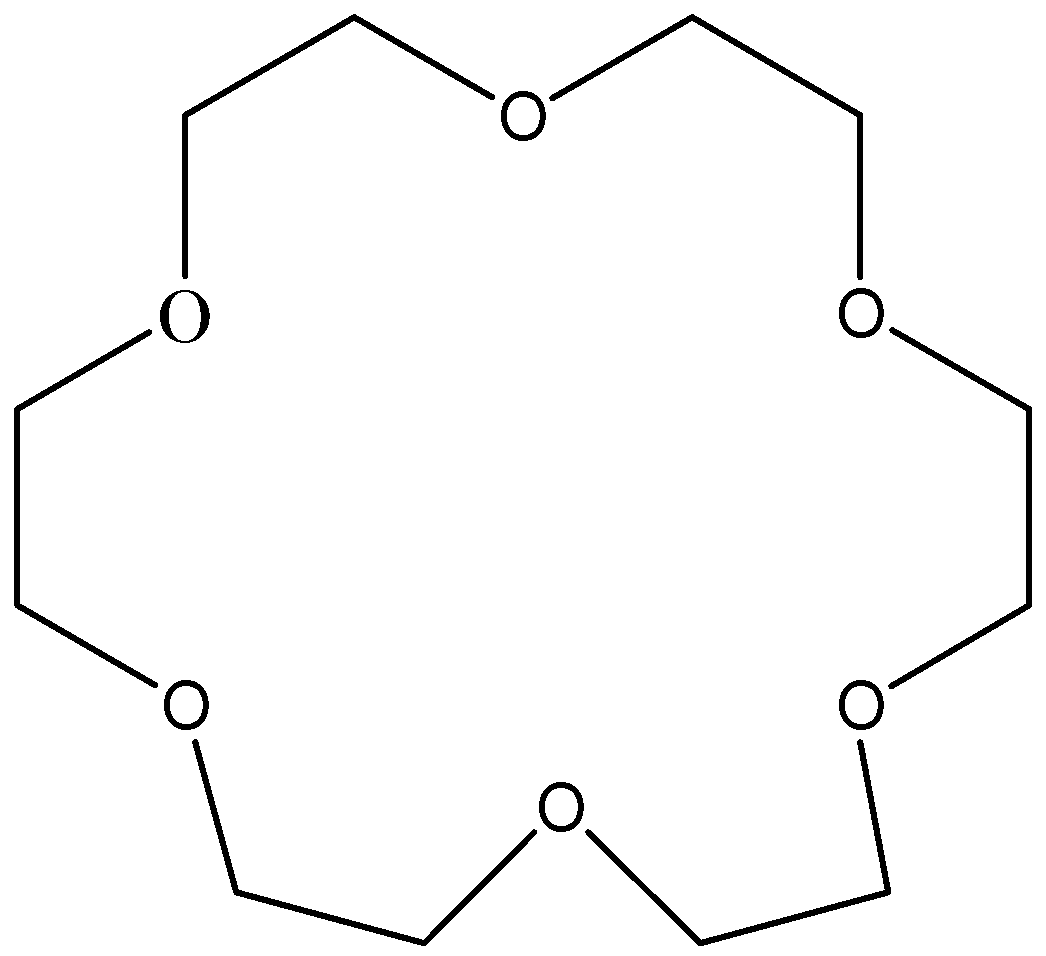
Therefore, the option B is correct.
Note:
After solving this question, we need to know that crown ethers are used to bring inorganic catalysts into the organic phase and to increase the solubility of inorganic compounds in organic solvents to promote chemical reactions. So changing the numbers of C and O atoms does indeed affect the properties of crown ethers.
Complete step by step answer:
We know that 18-Crown-6 has the ability to solubilize metal salts, and particularly potassium salts, in non-polar and dipolar aprotic solvents. So, it is widely used as a phase transfer catalyst. We can also use it as a metal complex agent in the preparation of a variety of molecular complexes. It is also used as phase-transfer catalysts.
The chemical formula of the organic compound 18-Crown-6 is ${[{C_2}{H_4}O]_6}$ and the IUPAC name is 1, 4, 7, 10, 13, 16-hexaoxacyclooctadecane. This organic compound is found as a white, hygroscopic crystalline solid with a low melting point.
So, to derive the number of an oxygen atom in a crown ether 18-crown-6 is 6. It is represented by the number of an oxygen atom and here, 18 represents the sum of the total number of carbon and oxygen atoms in the ether. Therefore, the number of an oxygen atom in 18-crown-6 is 6.
We can write the Structure of 18-crown-6:

Therefore, the option B is correct.
Note:
After solving this question, we need to know that crown ethers are used to bring inorganic catalysts into the organic phase and to increase the solubility of inorganic compounds in organic solvents to promote chemical reactions. So changing the numbers of C and O atoms does indeed affect the properties of crown ethers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




