
If the edge length of a KCl cell is 488pm, what is the length of KCl bond if it is crystallized in the FCC structure?
(A) 122 pm
(B) 244 pm
(C) 488 pm
(D) 974 pm
Answer
583.2k+ views
Hint: The FCC crystal lattice involves atoms situated at the corner of the cube and at the faces of the cube. The edge length in FCC lattice of KCl shows the distance between the two Cl atoms.
Complete step by step solution:
We are given that KCl is crystallized in FCC structure. FCC stands for face centered cubic lattice.
- In FCC lattice, the atoms are situated at the corners of the cube as well as in the centre of the faces of the cube. We can say that chlorine ions are bigger in size because they have a complete octet configuration. So, they will occupy the corners of the cube. The potassium ions will occupy the center of the faces of the cube.
- The figure of how KCl crystal lattice with FCC arrangement from a face of a cube is shown below.
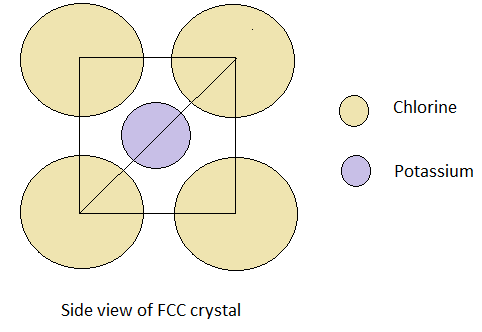
Here, we can see that the edge of the FCC crystal is the distance between the two chlorine atoms.
- The bond length is the distance between the two atoms forming the bond. So, here we need to find the distance between the K and Cl atom.
Thus, we can write from the figure that ${\text{Bond length = }}\dfrac{{{\text{Length of edge}}}}{2} = \dfrac{{488}}{2} = 244pm$
So, we can say that the bond length of KCl will be 244pm.
Therefore the correct answer is (B).
Note: If we are asked the distance between the K and Cl atoms in any other unit of length, then we can convert it using the fact that $1pm = {10^{ - 12}}m$ and $1pm = {10^{ - 1}}\mathop A\limits^0 $ . Do not get confused between FCC and BCC lattice.
Complete step by step solution:
We are given that KCl is crystallized in FCC structure. FCC stands for face centered cubic lattice.
- In FCC lattice, the atoms are situated at the corners of the cube as well as in the centre of the faces of the cube. We can say that chlorine ions are bigger in size because they have a complete octet configuration. So, they will occupy the corners of the cube. The potassium ions will occupy the center of the faces of the cube.
- The figure of how KCl crystal lattice with FCC arrangement from a face of a cube is shown below.

Here, we can see that the edge of the FCC crystal is the distance between the two chlorine atoms.
- The bond length is the distance between the two atoms forming the bond. So, here we need to find the distance between the K and Cl atom.
Thus, we can write from the figure that ${\text{Bond length = }}\dfrac{{{\text{Length of edge}}}}{2} = \dfrac{{488}}{2} = 244pm$
So, we can say that the bond length of KCl will be 244pm.
Therefore the correct answer is (B).
Note: If we are asked the distance between the K and Cl atoms in any other unit of length, then we can convert it using the fact that $1pm = {10^{ - 12}}m$ and $1pm = {10^{ - 1}}\mathop A\limits^0 $ . Do not get confused between FCC and BCC lattice.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




