
Identify the unsaturated compound from the following
(a)Propane
(b)Propene
(c)Propyne
(d)Chloropropane
(1)(a) and (b)
(2)(b) and (d)
(3)(c) and (d)
(4)(b) and (c)
Answer
582.9k+ views
Hint: Saturated hydrocarbons contain carbon-carbon single bonds whereas unsaturated hydrocarbons contain carbon-carbon double bonds or triple bonds. A single bond includes a sigma bond and a double bond includes both sigma and pi bonds.
Complete step by step answer:
a.Propane
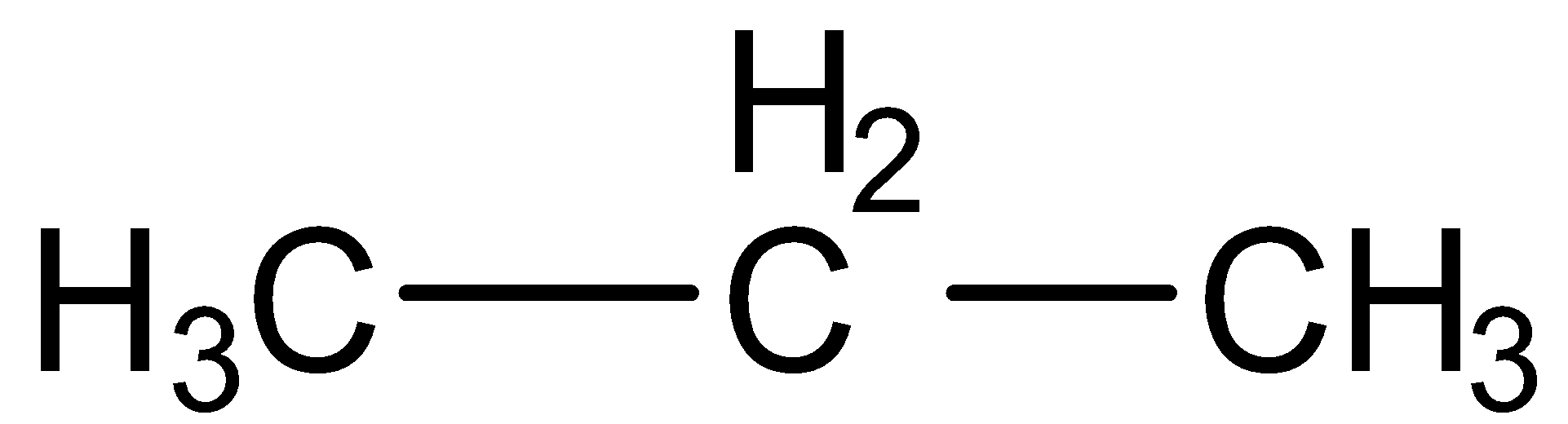
Here carbon-carbon single bond present, thus propane is a saturated hydrocarbon.
b.Propene
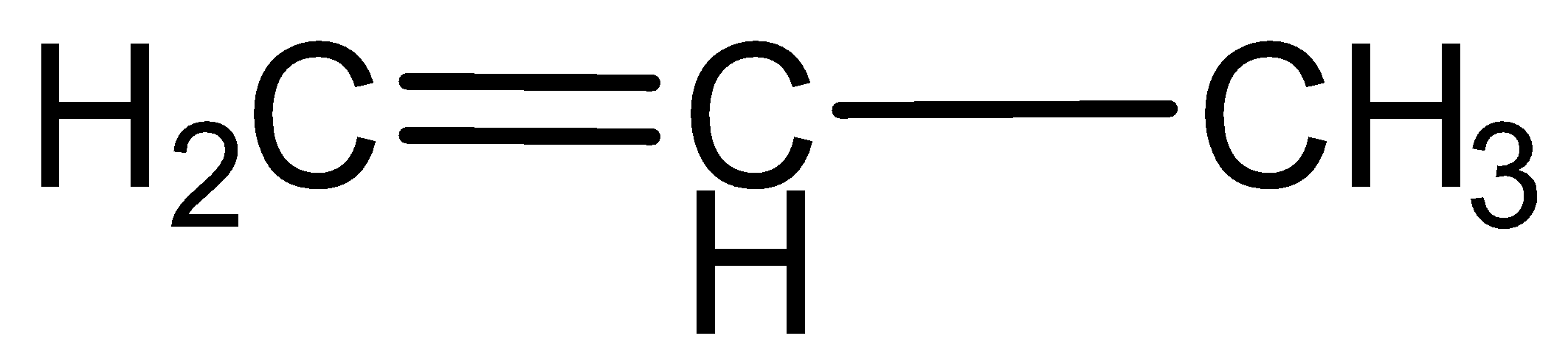
Here hydrocarbons contain carbon- carbon double bonds, which indicate that this is an unsaturated hydrocarbon.
c.Propyne
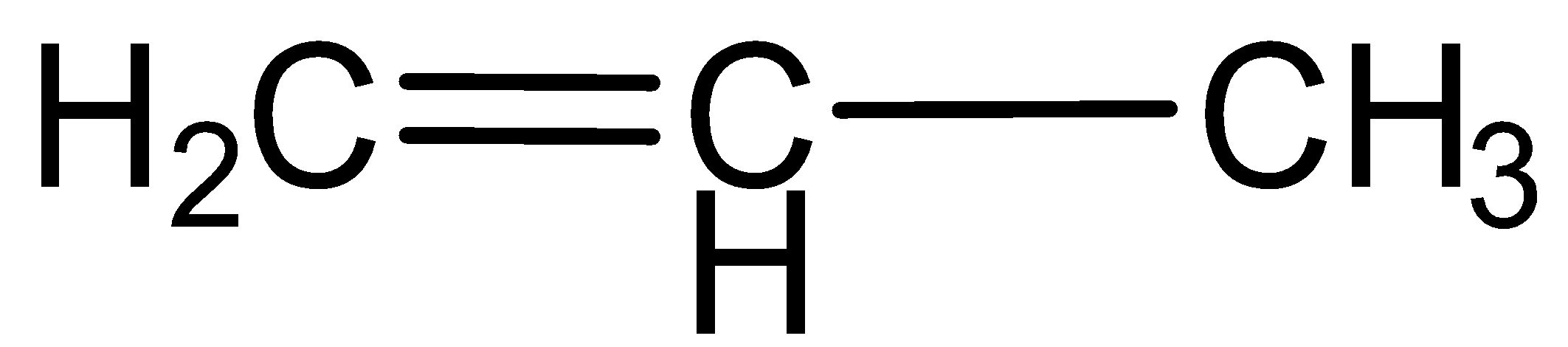
Here carbon- carbon triple bond is present, this is also an unsaturated hydrocarbon.
d.Chloropropane
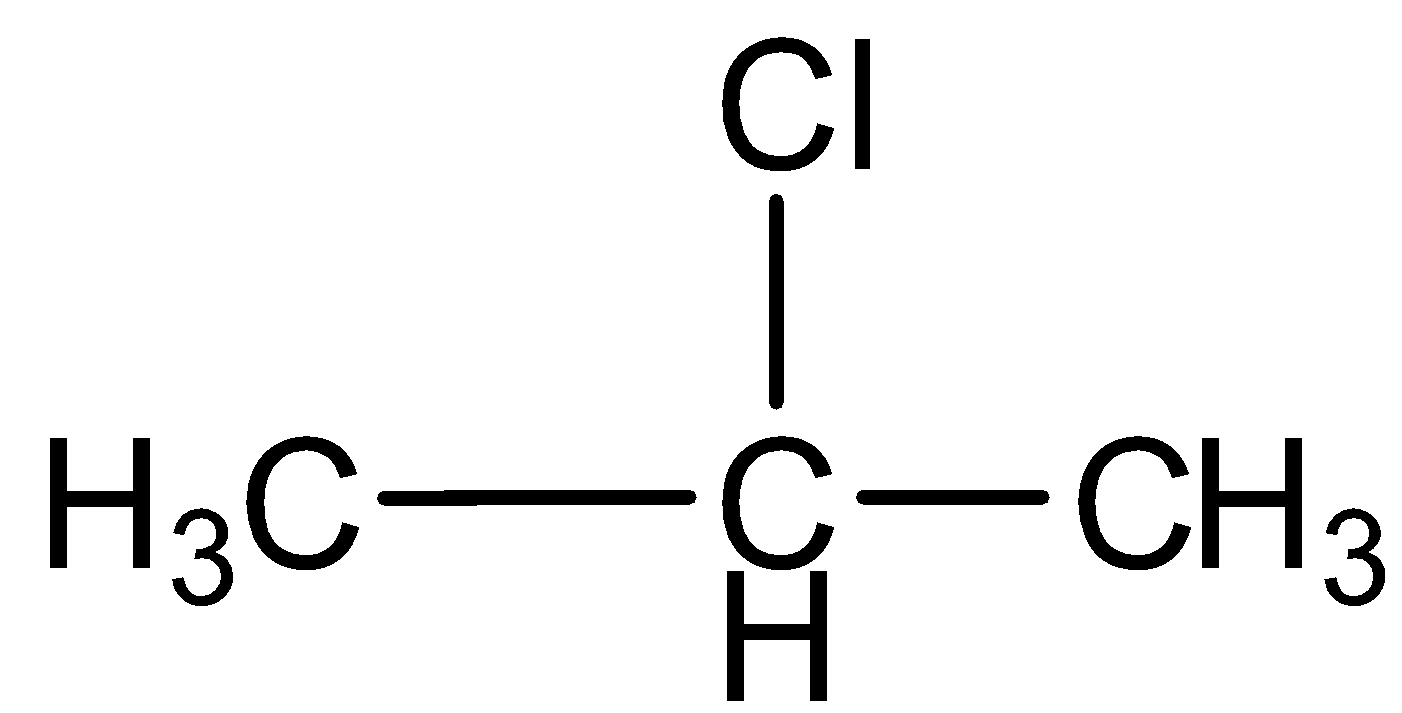
This compound contains a carbon- carbon single bond, so the compound is saturated hydrocarbon.
Therefore, the correct option is (4) (b) and (c).
Additional information:
The structural formulas of alkanes contain four type of carbon atoms:
(1)A carbon atom which is attached to one another is primary carbon.
(2)A carbon atom which is attached to the other two carbon atoms is called secondary carbon.
(3)A carbon atom which is attached to the other three carbon atoms is called tertiary carbon.
(4)A carbon atom which is attached to other four carbon atoms is called quaternary carbon.
Note:The hydrogen atoms in organic compounds may be classified as primary, secondary, tertiary, allylic or benzylic. There are a total six primary hydrogens and two secondary hydrogens in propane; the ratio of primary to secondary hydrogen in propane is $6/2$ or $3/1$.There is a degree of selectivity in hydrogen abstraction, secondary hydrogen is abstract faster than primary hydrogen.
Complete step by step answer:
a.Propane
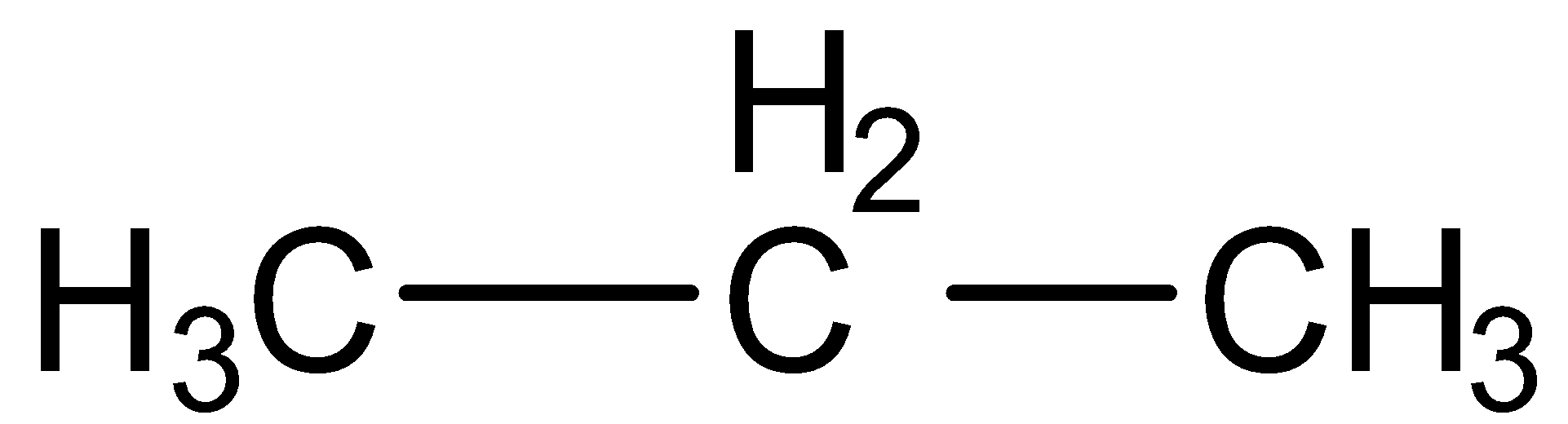
Here carbon-carbon single bond present, thus propane is a saturated hydrocarbon.
b.Propene

Here hydrocarbons contain carbon- carbon double bonds, which indicate that this is an unsaturated hydrocarbon.
c.Propyne

Here carbon- carbon triple bond is present, this is also an unsaturated hydrocarbon.
d.Chloropropane

This compound contains a carbon- carbon single bond, so the compound is saturated hydrocarbon.
Therefore, the correct option is (4) (b) and (c).
Additional information:
The structural formulas of alkanes contain four type of carbon atoms:
(1)A carbon atom which is attached to one another is primary carbon.
(2)A carbon atom which is attached to the other two carbon atoms is called secondary carbon.
(3)A carbon atom which is attached to the other three carbon atoms is called tertiary carbon.
(4)A carbon atom which is attached to other four carbon atoms is called quaternary carbon.
Note:The hydrogen atoms in organic compounds may be classified as primary, secondary, tertiary, allylic or benzylic. There are a total six primary hydrogens and two secondary hydrogens in propane; the ratio of primary to secondary hydrogen in propane is $6/2$ or $3/1$.There is a degree of selectivity in hydrogen abstraction, secondary hydrogen is abstract faster than primary hydrogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




